One mole of an ideal gas in a closed system, initially at 25°C and 10 bar, is first expanded adiabatically, then heated isochorically to reach a final state of 25°C and 1 bar. Assuming these processes are mechanically reversible, compute T and P after the adiabatic expansion, and compute Q, W, ?U, and ?H for each step and for the overall process. Take 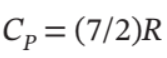 and
and 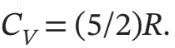
What will be an ideal response?
This is a two step process. First state the given information, 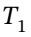 = 298.15 K and
= 298.15 K and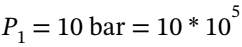 Pa. At these conditions, the molar volume is
Pa. At these conditions, the molar volume is 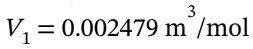 (from V = RT/P).
(from V = RT/P).
If the final state is 25 °C = 298.15 K and 1 bar = 1 * 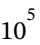 Pa, then the molar volume at the final state is
Pa, then the molar volume at the final state is 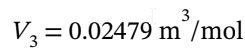 (from V = RT/P).
(from V = RT/P).
This is also the molar volume at the intermediate state, since the gas goes from the intermediate state to the final state at constant volume. We know that Q = 0 due to it being adiabatic which leads to the relation
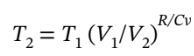 =118.7 K. Knowing
=118.7 K. Knowing 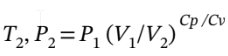 =0.398 bar.
=0.398 bar.
So for the adiabatic expansion, Q = 0, ?U = W = 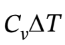 = 2.5 R*(118.7 K – 298.15 K) = -3729 J/mol, and ?H =
= 2.5 R*(118.7 K – 298.15 K) = -3729 J/mol, and ?H =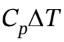 = 3.5 R*(118.7 K – 298.15 K) = -5221 J/mol.
= 3.5 R*(118.7 K – 298.15 K) = -5221 J/mol.
For the isochoric heating, ?U = 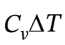 = 2.5 R*(298.15 K – 118.7 K)= 3729 J/mol, ?H =
= 2.5 R*(298.15 K – 118.7 K)= 3729 J/mol, ?H =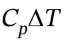 = 3.5 R*(298.15 K – 118.7 K)) = 5221 J/mol. At constant volume, W = 0, so Q = ?U = 3729 J/mol.
= 3.5 R*(298.15 K – 118.7 K)) = 5221 J/mol. At constant volume, W = 0, so Q = ?U = 3729 J/mol.
For the overall 2-step process, we then have ?U = 3729 J/mol, ?H = 5221 J/mol, Q = 3729 J/mol, and W = -3729 J/mol.
You might also like to view...
25 ft/sec? = 40 mi/hr
Answer the following statement true (T) or false (F)
Which of the following is needed for a noncontact tachometer to read the speed of a blower or pulley?
a. A calculator for the relevant equation b. Near darkness c. A reflective spot or piece of tape d. An infrared receiver
The safety professional’s responsibility in the safety and health program is to do the necessary housekeeping chores everyday.