The gas phase reaction A + B ? C is carried out in a continuous, steady state reactor. Which of the following is NOT a valid way to increase the fraction of A and B that are converted to C at equilibrium?
A. Increase the pressure.
B. Change the temperature such that the equilibrium constant of the reaction is higher.
C. Add a catalyst to the reactor that will accelerate the reaction.
D. Add a solvent to the reactor, in which C is highly soluble and A and B are slightly soluble.
E. All of the above are valid.
A. Incorrect. This applies Le Chatelier’s principle since the right hand side of the equation represents fewer moles than the left hand side.
B. Incorrect. This is a natural modification to improve conversions.
C. Correct. A catalyst can be used to increase the rate of a reaction, but will not influence the equilibrium constant.
D. Incorrect. This is a clever application of Le Chatelier’s principle. Because C is so soluble in the solvent, it is removed from the gas phase. This disrupts the equilibrium of the reaction, which responds by converting more A and B to C.
E. Incorrect. One of these will not work.
?
You might also like to view...
Many modern vehicles are equipped with a ____ system that is used to lock and unlock the doors, turn on the interior lights, and release the trunk latch prior to approaching the vehicle.
A. remote keyless entry B. wireless entry C. remote starter D. remote access
Once concrete is placed, curbing forms are removed after _____
a. 8 hours b. 16 hours c. 24 hours d. 48 hours
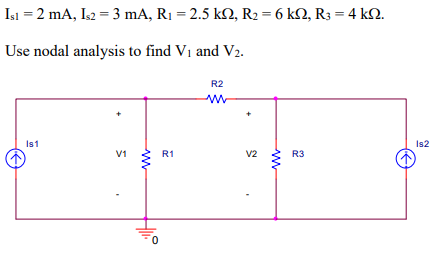
In the circuit shown below, let
Especially in the cattle industry, _______ has become popular for high volume breeding stock.
Fill in the blank(s) with the appropriate word(s).