It is a well-known fact that water has a higher specific heat than iron. Now, consider equal masses of water and iron that are initially in thermal equilibrium. The same amount of heat, 30 calories, is added to each one. Which statement is true?
A) They remain in thermal equilibrium.
B) They are no longer in thermal equilibrium; the iron is warmer.
C) They are no longer in thermal equilibrium; the water is warmer.
D) It is impossible to say without knowing the exact mass involved.
E) It is impossible to say without knowing the exact specific heats.
B
You might also like to view...
When viewed through a small telescope, galaxies appear bright, with a definitive edge.
Answer the following statement true (T) or false (F)
A rope pulls on the lower block in the figure with a tension force of 20 N. The coefficient of kinetic friction between the lower block and the surface is 0.16
The coefficient of kinetic friction between the lower block and the upper block is also 0.16. The pulley has no appreciable mass or friction. What is the acceleration of the 2.0 kg block? A) 4.1 m/s2 B) 5.1 m/s2 C) 8.4 m/s2 D) 9.2 m/s2
Which is the most common type of spacecraft propulsion used today?
A) solar sails B) nuclear rockets C) ion engines D) chemical rockets
Radioactivity Basics: Starting with 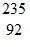 U, assume that the following three decays occur in sequence:(1) alpha decay(2) beta-minus decay(3) alpha decayDetermine the correct isotope that is left after each decay.
U, assume that the following three decays occur in sequence:(1) alpha decay(2) beta-minus decay(3) alpha decayDetermine the correct isotope that is left after each decay.
What will be an ideal response?