Heat Engines: A certain automobile engine takes in 4.00 kJ of heat and performs 1.10 kJ of mechanical work in each cycle.(a) Calculate the engine's efficiency.(b) How much heat is "wasted" in each cycle?
What will be an ideal response?
(a) 27.5% (b) 2.90 kJ
You might also like to view...
Vegetable oil is boiled by passing steam at 215ºF through a 1-inch ID by 1.25-inch OD steel tube. Predict the overall heat transfer coefficient based on the outside area of the tube if the convective heat transfer coefficients are 25 Btu/hr?ft^2?ºF for steam and 15 Btu/hr?ft^2 ?ºF for the vegetable oil. Neglect fouling factors.
What will be an ideal response?
The _________electrode is a 1-molar solution of hydrogen ions (H+ ) in HCl in contact with hydrogen gas at 1 atmosphere of pressure.
Fill in the blank(s) with the appropriate word(s).
The figure shows a distance vs. time graph of an object with three distinct regions, I, II, and III
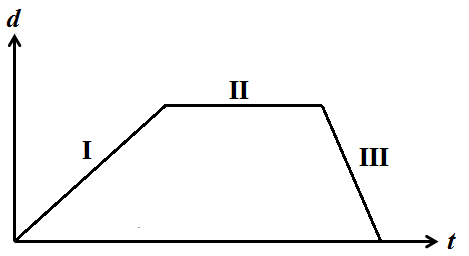 ?
?
The object is never at rest.
a.
True
b.
False
An electron in a hydrogen atom has orbital quantum number l = 4. How many possible values of the magnetic quantum number ml could it have?
A) 4 B) 9 C) 10 D) 3 E) 5