In the chemistry chapters presented in your
textbook, AM refers to?
a) the average mass of all elements taking part in a given reaction.
b) the atomic mass of a particular element.
c) the total mass present after a reaction has occurred.
d) the time during the day prior to 12 o'clock noon.
b
You might also like to view...
If a star is in hydrostatic equilibrium
A. its radiation pressure outwards and gravitational forces inwards are in balance. B. it is generating energy at the same rate everywhere. C. it is near the end of its life. D. it is in a stable binary orbit. E. it must be losing mass.
A radioactive isotope that emits a gamma quantum will change in what respect?
a. Atomic number increases by one. b. Atomic mass number decreases by one. c. Atomic number decreases by one. d. None of these choices are valid.
Determine the temperature distribution through a uranium slab shown. Assume energy generation of 4,500 Btu/min?ft^3 and the slab is surrounded by water at 190ºF with a convective heat transfer coefficient of 450 Btu/hr?ft^2 ?ºF. Use a value of 21.96 Btu/hr?ft?ºF for thermal conductivity of uranium.
What will be an ideal response?
RLC Circuits: The figure shows a series ac circuit. The inductor has a reactance of 80 ? and an inductance of 220 mH. A 30-? resistor and a capacitor whose reactance is 80 ? are also in the circuit, and the rms current in the circuit is 2.0 A. What is the capacitance of the capacitor?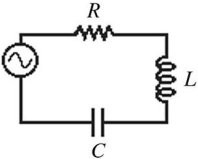
A. 34 ?F B. 37 ?F C. 32 ?F D. 30 ?F E. 27 ?F