An aqueous solution has a hydrogen concentration [H+] of 2.85 × 10-1. Find the concentration of hydroxl ions [OH-] in the solution.
A. 3.51 × 10-14 mol/L OH-
B. 3.51 × 1014 mol/L OH-
C. 3.51 × 10-13 mol/L OH-
D. 2.85 × 10-14 mol/L OH-
Answer: A
Mathematics
You might also like to view...
Use scientific notation to solve the problem.A light-year is the distance that light travels in one year. Find the number of miles in a light-year if light travels 1.86 x 105 miles per second.
A. 6.0 x 105 miles B. 6.0 x 1014 miles C. 6.0 x 107 miles D. 6.0 x 1012 miles
Mathematics
Convert the units.9.84 m =  cm
cm
A. 98.4 cm B. 984 cm C. 0.984 cm D. 0.0984 cm
Mathematics
Solve the given equation for the indicated variable.M = ?r2hd for r
A. r = 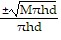
B. r = 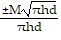
C. r = 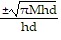
D. r = ±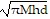
Mathematics
Suppose 35 points are placed around a circle. A line segment is drawn between each pair of points. How many line segments are drawn?
a. 1,190 b. 1,225 c. 630 d. 35 e. 595
Mathematics