If the overall mixture is 80% by mole component 1, will the mixture exist in one phase or multiple phases in equilibrium at 327 K? If the mixture does exist in multiple phases, what is the composition of the equilibrium phases?
Consider the following figure that describes the solid-liquid equilibrium of a binary mixture:
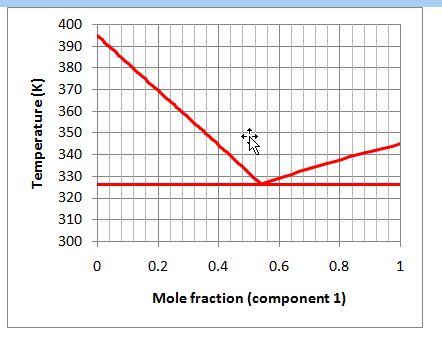
A. 1 solid phase
B. 1 liquid phase (x1 = 0.80) and 1 solid phase (z1 = 1.0)
C. 1 liquid phase (x1 = 0.54) and 2 solid phases (z1? = 1.0; z2? = 1.0)
D. 2 solid phases (z1? = 1.0; z2? = 1.0)
E. None of the above.
A. Incorrect. Since the solid is immiscible, one solid phase will occur only when the system is pure (of either component) and not a mixture.
B. Incorrect. Review how to read a graph like this from the text.
C. Correct. This point is on the SLLE line, so all three phases will exist at once at the compositions provided. This is the eutectic.
D. Incorrect. If the point were below the solidus line, this would be correct, but it is not. It is at the eutectic temperature.
E. Incorrect. One of the above is correct.
?
You might also like to view...
When constructing ceiling systems, fire-rated and multilayer assemblies require the use of _____.
a. slip connectors b. wire ties c. lightweight studs d. welded joints
What kinds of glass were preferred for use in greenhouses before the 20th century? What types weren’t
used, and why?
What will be an ideal response?The pressure in a gasoline engine combustion chamber can be as high as ______
A) 1000 PSI B) 400° C) Both A and B D) Neither A nor B
A cooling system bleeder valve is used to ________
A) Release air from the system when refilling B) Bleed out any brake fluid that may have gotten into the cooling system C) Remove excess coolant D) Control system pressure