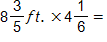 ?
?
A.
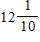 ft.
ft.B.
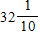 ft.
ft.C.
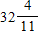 ft.
ft.D.
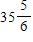 ft.
ft.Answer: D
You might also like to view...
Answer the following statement(s) true (T) or false (F)
A. For an exothermic reaction, the equilibrium constant decreases with increasing temperature. B. When the Gibbs free energy of a reaction is negative, the equilibrium constant will be greater than 1. C. The shortcut van ’t Hoff equation assumes ?CP = 0. D. For a gas phase reaction, if the number of moles of reactant and the number of moles of product in a reaction are identical, then the extent of reaction at equilibrium will be completely independent of pressure. E. The equilibrium constant KP is a function of temperature.
____ is used in tips having eight preheat holes or in a two-piece tip that is not recessed.
A. MPS gas B. Acetylene C. Natural gas D. Propane
A term used to describe flow in a heat exchanger where shell-side flow vertically crosses the horizontal tube flow.
a. cross flow b. horizontal flow c. parallel flow d. counter flow
The political developments of the l890s were largely shaped by
A. the widespread prosperity and federal budget surpluses. B. America's growing involvement in overseas conflicts. C. the most severe and extended economic depression up to that time. D. the growing black rebellion against segregation and racial oppression. E. the deadlock among Republicans, Democrats, and Populists in Congress.