Which of the following does not result from applying the Schrödinger equation to the electron in the hydrogen atom?
e.
This statement is not true. Goudsmit and Uhlenbeck proposed that the electron have an intrinsic spin quantum number of s = 1/2. Schrödinger's equation fully solved did not include this quantum number.
You might also like to view...
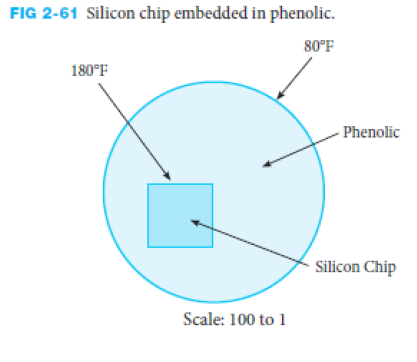
Using graphical methods, estimate the temperature distribution through the phenolic disk surrounding the silicon chip sketched in Figure 2-61. Then estimate the heat transfer per unit depth.
This figure shows an airplane traveling east to west along Earth's equator at the same speed that Earth rotates from west to east. People on Earth would, of course, simply see the airplane fly westward at 1,670 km/hr. What is the significance of the difference in viewpoints between people on Earth and people on the Moon?
A) It shows that motion is relative not absolute. B) It shows that you can get a wrong answer if you don't view the situation from the correct perspective. C) It shows that the airplane is traveling in a direction opposite to Earth's rotation. D) It shows that airplanes can move quite fast if you view them from the right place.
A person looks horizontally at the edge of a swimming pool. If its length is 5 m, and the pool is filled to the surface, to what depth (in m) could the observer see? (n for water is 1.33)
a. 3.2 b. 4.4 c. 2.1 d. 1.0 e. 0.3
When a positive charge is released and moves along an electric field line, it moves to a position of
a. lower potential and lower potential energy. b. lower potential and higher potential energy. c. higher potential and lower potential energy. d. higher potential and higher potential energy. e. greater magnitude of the electric field.