The vapor pressure of benzene at T=25 °C is 0.125 bar. The Appendix of your book contains enough additional data on benzene to estimate other vapor pressures by the Antoine, shortcut, or Clausius-Clapeyron approaches.
The molar volume of liquid benzene at room temperature and pressure (T=25 °C, P=1 bar) is V = 88.9 cm3/mol. Liquid benzene also has a coefficient of thermal expansion of
? =1.237 x 10-3 K-1and isothermal compressibility of ? = 8.9 x 10-5bar-1
A) Give your best estimate of the vapor pressure of benzene at T=50 °C, and explain why you chose the method that you chose.
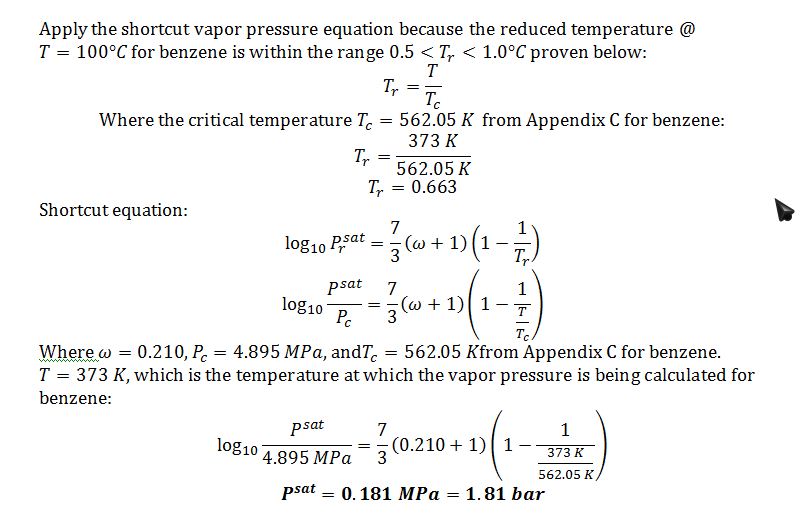
B) Give your best estimate of the vapor pressure of benzene at T=100 °C, and explain why you chose the method that you chose.
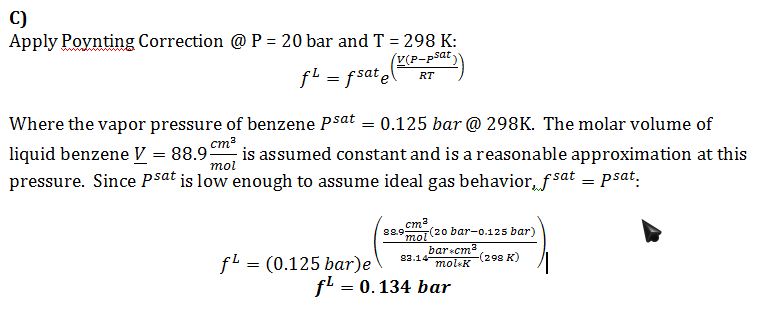
C) Give your best estimate of the fugacity of liquid benzene at T=25 °C and P=20 bar, and state any assumptions that you make.
D) (10 points) Benzene expands as it’s heated. Estimate the molar volume of liquid benzene at a temperature of 50 °C and a pressure of P=1 bar.
A)
Apply Antoine equation since it is a good model for vapor pressure when 20°C
?log?_10 P^sat=A-B/(T+C)
Where T is the temperature expressed in Celsius, and P^sat is expressed in mmHg.
From Appendix E: A=6.90565, B=1211.033, C=220.790
?log?_10 P^sat=6.90565-1211.033/(50+220.790)
P^sat=271.3 mmHg
You might also like to view...
Your smartphone touch screen implements a 100 x 100 switch array. What is the minimum number of bits that defines the address of each switch assuming separate row and column addresses?
What will be an ideal response?
Tenga precaución con el manejo de las roscas de tubería recién cortadas debido a que:
a. tienen protuberancias. b. son frágiles. c. son resbaladizos por el lubricante. d. son filosas.
____________________ are loads found in many systems and wiring diagrams that are responsible for taking electric energy and convert it to heat.
Fill in the blank(s) with the appropriate word(s).
Solve and round to 1 decimal place: (26.30 - 17.80) × (0.950 + 3.87).
Fill in the blank(s) with the appropriate word(s).