The pH of a solution is given by the formula 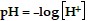 , where
, where 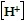 is the concentration of hydrogen ions in moles per liter in the solution. Use this formula to solve the problem.Lower levels of pH indicate a more acidic solution. If the pH of solution A is 4.2 and the pH of solution B is 6.9, how much more acidic is solution A than solution B?
is the concentration of hydrogen ions in moles per liter in the solution. Use this formula to solve the problem.Lower levels of pH indicate a more acidic solution. If the pH of solution A is 4.2 and the pH of solution B is 6.9, how much more acidic is solution A than solution B?
A. 0.0000630 times as acidic
B. 501.2 times as acidic
C. -0.0000630 times as acidic
D. 0.00200 times as acidic
Answer: B
You might also like to view...
Provide an appropriate response.Consider the velocity field F, which has for every z the configuration shown.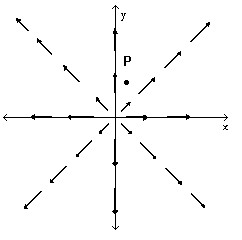 Is the divergence at p positive, negative, or zero?
Is the divergence at p positive, negative, or zero?
A. positive B. negative C. zero
Rewrite the expression using a determinant. Show all your work.
What will be an ideal response?
Provide an appropriate response.When using the substitution or elimination method to solve a system of two equations, you end up with an equation stating 0 = 0. What does this indicate to you about the system of equations?
What will be an ideal response?
Solve the problem.If h(x) = x2 - 3x + 2 , solve h(x) > 0.
A. (-?, 1) B. (1, 2) C. (2, ?) D. (-?, 1) or (2, ?)