Use the pH scale to solve the problem. Negative ions, designated by the notation [OH-] are always present in any acid or base. The concentration, [OH-], of these ions is related to [H+] by the equation [OH-] ? [H+] = 10-14 moles per liter. Find the concentrations of [OH-] and [H+] in moles per liter for the substance with the following pH value. Round to two decimal places. pH = 7.3
A. [H+] = 5.01 × 10-8
[OH-] = 2.00 × 10-7
B. [H+] = 2.00 × 10-8
[OH-] = 5.01 × 10-8
C. [H+] = 5.01 × 10-6
[OH-] = 2.00 × 10-8
D. [H+] = 2.00 × 10-7
[OH-] = 5.01 × 10-8
Answer: A
Mathematics
You might also like to view...
What is the resistance of a circuit having a conductance of 11.7 ð 10-6 S?
a. 8.55 ohms b. 855 ohms c. 85.5 kohms d. 11.7 kohms e. 11.7 megohms
Mathematics
Perform the operation indicated. Simplify.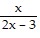 -
- 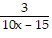
A. 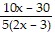
B. 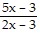
C. 
D. 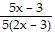
Mathematics
Write the equation in exponential form.log1/464 = -3
A. 641/4 = 3
B. ( )3 = 64
)3 = 64
C. (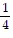 )-3 = 64
)-3 = 64
D. (-3)1/4 = 64
Mathematics
Underline the digit that occupies the given place.6.55 Hundredths place
Hundredths place
A. 6.5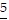
B. 6.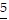 5
5
C. 6.55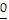
D. 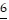 .55
.55
Mathematics