How have scientists used isotopic analysis to determine that the rise of atmospheric CO2 is mostly from the burning of fossil fuels?
A) 14C concentrations are much higher in urban areas than in rural areas, indicating fossil fuels must have a higher 14C/12C ratio than carbon from other sources.
B) Since 14C is an unstable isotope, scientists have dated the atmospheric carbon to the period since the Industrial Revolution.
C) Both coal and oil contain a high proportion of 14C. By analyzing 14C/12C ratios, scientists have been able to determine the source based on this fact.
D) The decrease in ratios of atmospheric 13C and 14C to 12C indicates that the source is fossil fuel carbon.
D
You might also like to view...
Describe the important steps and processes of the formation of precipitation in the Bergeron process, starting with a mixture of small ice crystals and supercooled water droplets and ending with the dropping of precipitation from a cloud
What will be an ideal response?
About how long was California a part of Mexico before it became a U.S. state?
A) 25 years B) 50 years C) 100 years D) 200 years
The daily (diurnal) range of temperature is greatest next to the ____________________
Which feature on this diagram is a cirque?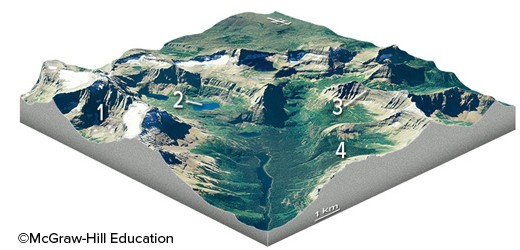
A. 1, the circular depression at the head of the valley B. 2, a lake in the upper valley C. 3, a steep ridge D. 4, a valley that is above the level of the main valley