The volume holding 0.63 mole of an ideal gas is compressed to 1/4 of its original volume by a piston. At the same time the pressure of the gas triples. If the original absolute temperature of the gas was T, that is the new temperature after compression?
a. 4/3T b. 3/4T
c. 0.63(3/4T) d. 0.63(4/3T)
b
You might also like to view...
Springs: The graphs shown show the magnitude F of the force exerted by a spring as a function of the distance x the spring has been stretched. For which one of the graphs does the spring obey Hooke's law?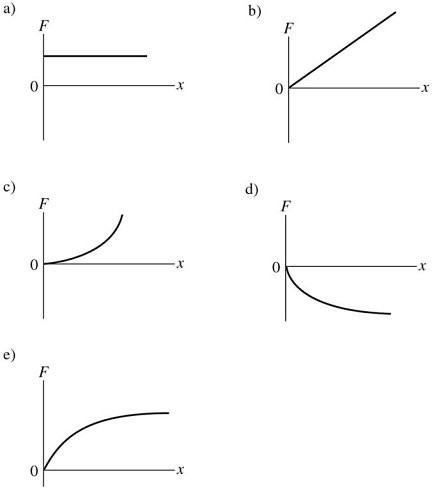
A. Graph a B. Graph b C. Graph c D. Graph d E. Graph e
What happens to a given volume of water when heated from 0°C to 4°C?
a. density increases b. density decreases c. density remains constant d. vaporizes
Which has a larger volume, a liter of water or a liter of mercury (a liquid metal)?
a. water b. mercury c. none of these
A thin rectangular piece of wood floats in water. You slowly pour oil with a density equal to that of the wood on the surface of the water until the height of the oil above the water is twice the height of the piece of wood. Which statement is correct?
a. The wood floats on top of the oil, so it sticks up in the air. b. The wood does not change its position c. The wood sinks below the surface of the water. d. The wood is half in the water and half in the oil. e. The wood floats in the oil just above the water.