An ideal gas starts in state A at temperature T. The gas expands to new volume V by an adiabatic process and its final temperature is T?. What is the relationship between T and T??
A)
T = T?
B)
T > T?
C)
T < T?
D)
The answer depends on the heat capacity of the ideal gas.
E)
The answer depends on the number of moles of gas and the pressure.
B
You might also like to view...
Energy Levels: The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength ? is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels?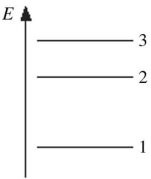
A. both ?/2 and ?/3 B. only ?/2 C. both 2? and 3? D. only 2?
According to Wein's law, the larger the blackbody, the shorter its peak wavelength
Indicate whether the statement is true or false
If the intensity level of one trombone is 70 dB, calculate a reasonable estimate for the intensity level of 76 trombones all playing simultaneously in the same place?
A) 146 dB B) 89 dB C) 70 dB D) 76 dB E) 82 dB
If y = 0.02 sin (30x ? 400t) (SI units), the angular frequency of the wave is
a. 30 rad/s. b. 30/2? rad/s. c. 400/2? rad/s. d. 400 rad/s. e. 40/3 rad/s.