Determine the Fourier series of the periodic function in Fig. 17.50.
 figure 1.png)
 figure 3.png)
 FIGURE 4.png)
 ANSWER.png)
You might also like to view...
Gas at constant T and P is contained in a supply line connected through a valve to a closed tank containing the same gas at a lower pressure. The valve is opened to allow flow of gas into the tank, and then is shut again.
(a) Develop a general equation relating 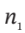 and
and 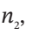 the moles (or mass) of gas in the tank at the beginning and end of the process, to the properties
the moles (or mass) of gas in the tank at the beginning and end of the process, to the properties 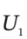 and
and 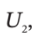 the internal energy of the gas in the tank at the beginning and end of the process, and H?, the enthalpy of the gas in the supply line, and to Q, the heat transferred to the material in the tank during the process.
the internal energy of the gas in the tank at the beginning and end of the process, and H?, the enthalpy of the gas in the supply line, and to Q, the heat transferred to the material in the tank during the process.
(b) Reduce the general equation to its simplest form for the special case of an ideal gas with constant heat capacities.
(c) Further reduce the equation of (b) for the case of 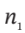 = 0.
= 0.
(d) Further reduce the equation of (c) for the case in which, in addition, Q = 0.
(e) Treating nitrogen as an ideal gas for which 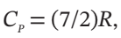 apply the appropriate equation to the case in which a steady supply of nitrogen at 25°C and 3 bar flows into an evacuated tank of 4
apply the appropriate equation to the case in which a steady supply of nitrogen at 25°C and 3 bar flows into an evacuated tank of 4 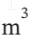 volume, and calculate the moles of nitrogen that flow into the tank to equalize the pressures for two cases:
volume, and calculate the moles of nitrogen that flow into the tank to equalize the pressures for two cases:
1. Assume that no heat flows from the gas to the tank or through the tank walls.
2. Assume that the tank weighs 400 kg, is perfectly insulated, has an initial temperature of 25°C, has a specific heat of 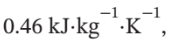 and is heated by the gas so as always to be at the temperature of the gas in the tank.
and is heated by the gas so as always to be at the temperature of the gas in the tank.
Who were the members of the official Triumvirate of 42–33 BCE?
a. Octavian, Caesar, and Antony b. Lepidus, Cicero, and Octavian c. Antony, Lepidus, and Caesar d. Lepidus, Octavian, and Antony
The way a large commercial customer uses power and the resulting system electrical efficiency is known a facility's __________________
A. real power B. apparent power C. volt-amps reactive power (VARS) D. power factor
Digital computers add only two binary numbers at a time
Indicate whether the statement is true or false