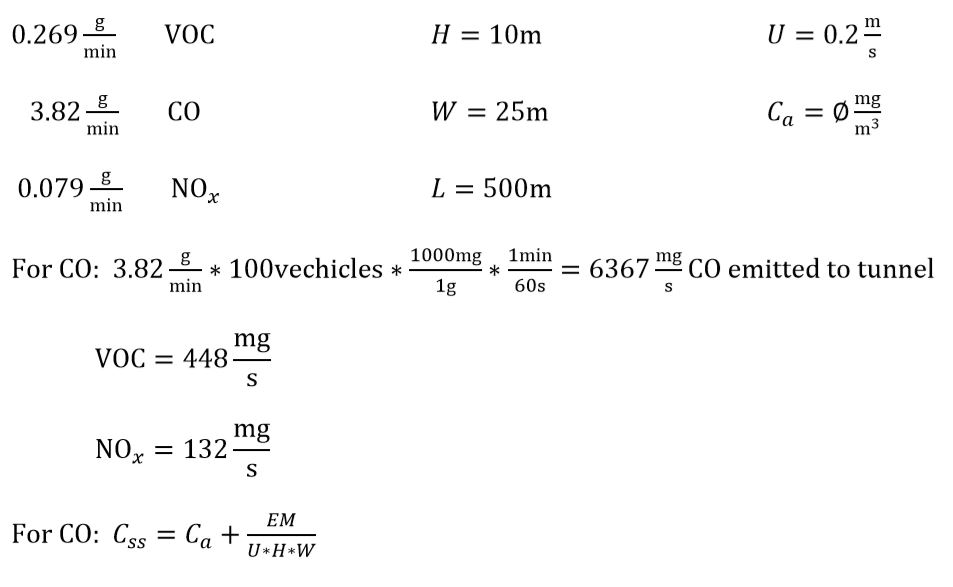
A traffic accident causes traffic to come to a halt in an underwater tunnel. The people in their vehicles allow their engines to idle. There are 100 vehicles stopped in the tunnel; assume their vehicle engines emit on average 0.269 g/min of VOC (aldehyde), 3.82 g/min CO, and 0.079 g/min NOx. The tunnel is long and narrow with a height of 10 m, a width of 25 m, and a length of 0.5 km. Assume the ambient air concentrations of CO, VOCs, and NOx are small. The mean air velocity through the tunnel, when traffic stops, is limited to 0.2 m/s.
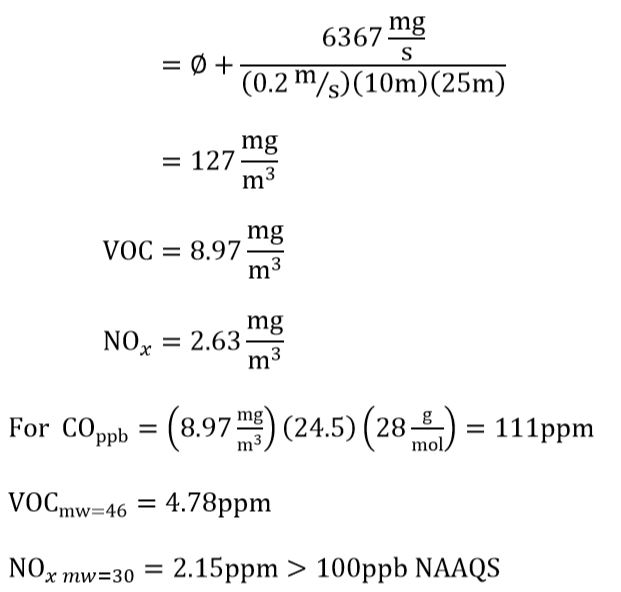
a. Assuming traffic remains halted long enough for the concentration carbon monoxide, nitric oxides, and hydrocarbons in the canyon to reach steady state, what would the expected concentrations of carbon monoxide, nitric oxides and hydrocarbons be in ppmv in the tunnel?
b. Do the CO and NOx concentrations exceed NAAQS?
c. Does the CO concentration exceed the OSHA’s permissible exposure limit (PEL)?
What will be an ideal response?
100 vehicles emitting:
You might also like to view...
What is glare?
What will be an ideal response?
Most biodiesel in the U. S. produced in 2012 came from ____
a. olive oil c. soybean oil b. canola oil d. peanut oil
Which of the following statements does NOT describe an advantage of digital technology?
A) Information storage is easy. B) The circuits are less affected by noise. C) The time it takes to process information is shorter. D) The operation can be programmed.
What are weather-tight connectors?
What will be an ideal response?