What is the importance of the fact that methane has a chemical formula CH4, while coal has a chemical formula approximately CH0.8?
What will be an ideal response?
We know that burning is the combining of a compound with oxygen to produce
water and carbon dioxide. The burning of these two compounds in oxygen results in
differing amounts of CO2 and H2O.
Balancing the chemical equations, we may write
CH4 + X O2 -> Y CO2 + Z H2O,
where X, Y, and Z represent numbers that we will determine. We see that there is only one
carbon on the left, so there must be just one carbon on the right, meaning that Y = 1 . There
are four hydrogen atoms on the left, which is the same as two hydrogen molecules, so there
must be four atoms or two molecules' worth of hydrogen on the right, which means that Z
= 2 . There are two oxygen atoms (one oxygen molecule) associated with the carbon on the
right, and another two oxygen atoms (one oxygen molecule) associated with the water on
the right, so there must have been two oxygens on the left, meaning that X = 2 . So
CH4 + 2O2 -> CO2 + 2H2O
and we use the same method to write
5CH0.8 + 6O2 -> 5CO2 + 2H2O.
It is clear that there is a greater ratio of CO2 to water in the exhaust from burning CH0.8.
You might also like to view...
There are three ________ of elementary particles in the standard model.
Fill in the blank(s) with the appropriate word(s).
The conversion of solar radiation into chemical energy by plants that releases oxygen is called
A) plant succession. B) photosynthesis. C) dendrochronology. D) deforestation. E) podzolization.
Matter Waves: A proton that is moving freely has a wavelength of 0.600 pm. (mproton = 1.67 × 10-27 kg, e = 1.60 × 10-19 C, h = 6.626 × 10-34 J ? s)(a) What is its momentum?(b) What is its speed?(c) What potential difference would it have been accelerated through, starting from rest, to reach this speed?
What will be an ideal response?
Which one of the following cannot be a statement of Gauss's law for some physical situation?
A.
B.
C.
 .
.D.
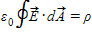 .
.E.
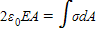 .
.