Use Bohr's model to draw a sodium (Na) atom and a chlorine (Cl) atom. Using your model, explain what happens when sodium reacts with chlorine to form table salt. Include in your explanation ion and ionic bond formation. 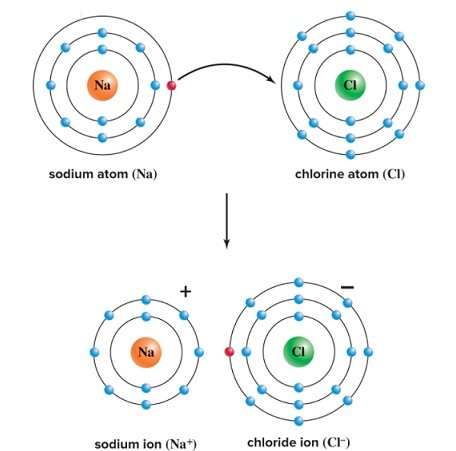
What will be an ideal response?
Sodium donates an electron to chlorine to form a sodium ion (Na+) and a chloride ion (Cl-). Ions are charged particles that have an unequal number of protons and electrons. These ions are oppositely charged and are, therefore, attracted to each other. The attraction between oppositely charged ions that were formed by a transfer of electrons is an ionic bond.
You might also like to view...
What is the update anomaly?
Starting points
A. are usually learned or inferred as negotiations get under way. B. are given up as concessions are made. C. are usually contained in the opening statements each negotiator makes. D. are not known to the other party.
The Great Man approach to effective leadership is about ______.
a. leadership traits b. leadership needs c. leadership behavioral styles d. neurological leadership profiles
Meridian Fashions uses standard costs for their manufacturing division. The allocation base for overhead costs is direct labor hours. From the following data, calculate the fixed overhead cost variance.
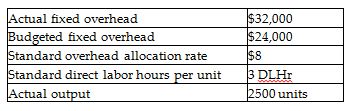
A) $36,000 F
B) $36,000 U
C) $8000 F
D) $8000 U