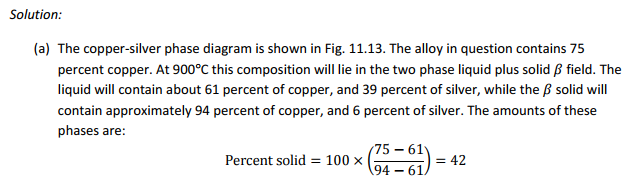
(a) A copper?75 percent silver alloy is slowly cooled from the liquid state to 900°C, and allowed to come to equilibrium. Estimate the amount and composition of both the liquid and solid phases.
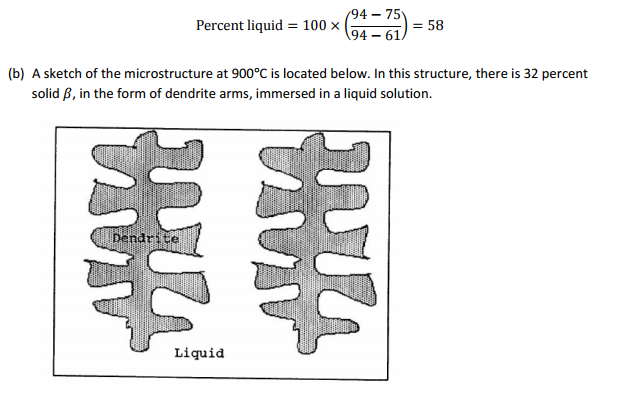
(b) Make a sketch of the 900°C equilibrium structure of the alloy.
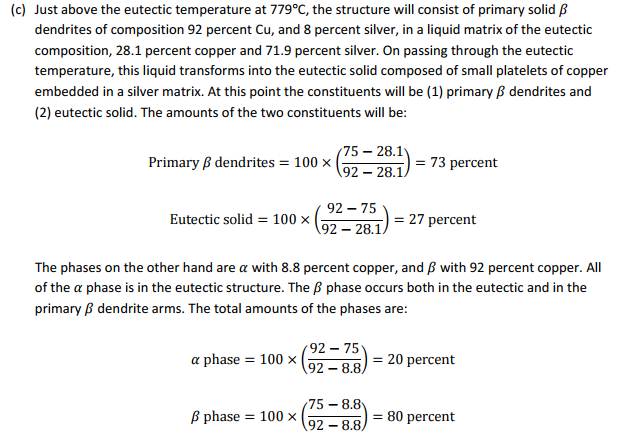
(c) Now assume the alloy is slowly cooled to just below the eutectic temperature. What are the weight percentages and compositions of the phases and constituents at this point?

You might also like to view...
Research and write a report of approximately 250 words describing a specific GIS application. Prepare a PowerPoint presentation of your research and present the slide show to your class or office.
What will be an ideal response?
What is the difference between boring and drilling?
What will be an ideal response?
A section that appears in an auxiliary view is known as an auxiliary section.
Answer the following statement true (T) or false (F)
Describe why plastic must be cleaned with both isopropyl alcohol and wax and grease remover.
What will be an ideal response?