A tank of 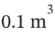 volume contains air at 25°C and 101.33 kPa. The tank is connected to a compressed-air line which supplies air at the constant conditions of 45°C and 1500 kPa. A valve in the line is cracked so that air flows slowly into the tank until the pressure equals the line pressure. If the process occurs slowly enough that the temperature in the tank remains at 25°C, how much heat is lost from the tank? Assume air to be an ideal gas for which
volume contains air at 25°C and 101.33 kPa. The tank is connected to a compressed-air line which supplies air at the constant conditions of 45°C and 1500 kPa. A valve in the line is cracked so that air flows slowly into the tank until the pressure equals the line pressure. If the process occurs slowly enough that the temperature in the tank remains at 25°C, how much heat is lost from the tank? Assume air to be an ideal gas for which 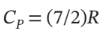 and
and 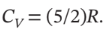
What will be an ideal response?
We can make a simple sketch of the situation as shown below.
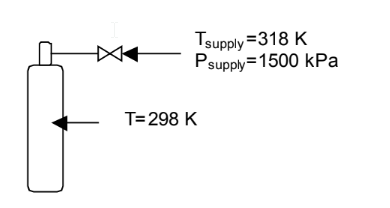
Now, we need to write mass (or mole) and energy balances on the gas in the tank. We will use a control volume that includes the gas inside the tank and extends just upstream of the valve (so the pressure and temperature of the gas entering the system are 1500 kPa and 318 K, respectively).
The mole balance on the gas in the tank is just
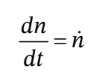
where 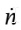 is the molar flow rate into through the valve (which need not be constant).
is the molar flow rate into through the valve (which need not be constant).
The corresponding energy balance is:
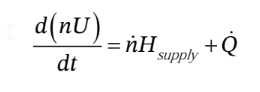
where 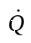 is the rate of heat addition to the system (it will be negative, since heat is removed from the system). The work term is zero, because no shaft work is done (there are no moving parts). We can expand the derivative on the left hand side using the product rule, and replace
is the rate of heat addition to the system (it will be negative, since heat is removed from the system). The work term is zero, because no shaft work is done (there are no moving parts). We can expand the derivative on the left hand side using the product rule, and replace 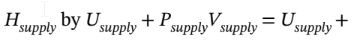
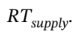 This gives
This gives
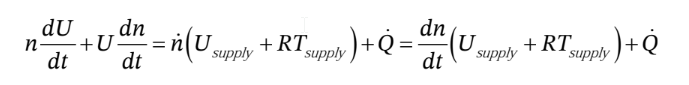
Where in the last expression we have used the mole balance to substitute 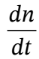 for
for 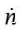 Now, since the temperature inside the cylinder remains constant, U also remains constant (for an ideal gas, U is only a function of T). So, the first term on the LHS is zero. Solving for
Now, since the temperature inside the cylinder remains constant, U also remains constant (for an ideal gas, U is only a function of T). So, the first term on the LHS is zero. Solving for 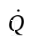 then leaves
then leaves
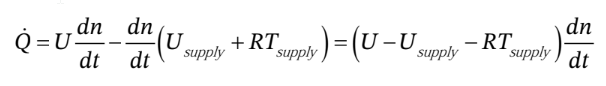
This says that the rate of heat addition is directly proportional to the rate at which gas flows into the cylinder. Note that everything inside the parenthesis on the RHS of the equation is constant. The total heat added is just the integral of this over the whole process.
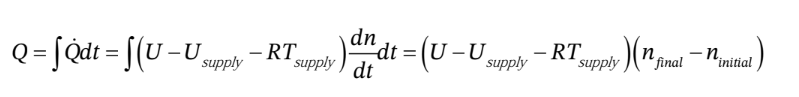
We also recognize the 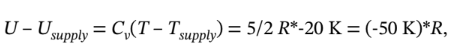 and
and 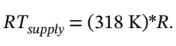 So, the RT term is much bigger than the
So, the RT term is much bigger than the 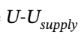 term.
term.

So, finally, 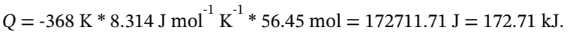
You might also like to view...
Which of the following is a benefit of prefabrication?
a. Onsite provides better working conditions. b. The shop provides better working conditions. c. Workload and overtime increases. d. Experienced technicians must complete all the work.
Explain the use of a simple building pressurization test.
What will be an ideal response?
China is the most populous country in the world
Indicate whether the statement is true or false
A square plot has a perimeter of 64 meters. How long is one side?
Fill in the blank(s) with the appropriate word(s).