If light has a wavelength of 600 nm in a vacuum, what is its wavelength in a material with an index of refraction of 1.50?
A. 600 nm
B. 900 nm
C. 400 nm
D. 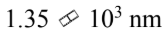
E. 267 nm
C. 400 nm
You might also like to view...
An Olympic athlete throws a javelin at four different angles above the horizontal, each with the same speed: 30°, 40°, 60°, and 80°. Which two throws cause the javelin to land the same distance away?
A) 30° and 80° B) 40° and 60° C) 30° and 40° D) 40° and 80° E) 30° and 60°
Calorimetry: A camper is about to drink his morning coffee. He pours 400 grams of coffee, initially at 75°C into a 250-g aluminum cup, initially at 16°C. What is the equilibrium temperature of the coffee-cup system, assuming no heat is lost to the surroundings? The specific heat of aluminum is 900 J/kg ? K, and the specific heat of coffee is essentially the same as that of water, which is 4186 J/kg ? K.
A. 45°C B. 62°C C. 65°C D. 68°C E. 71°C
Given the following generic chemical reaction, which is the reactant?
X ? Y A) Y is the reactant. B) X is the reactant. C) ? is the reactant. D) Both X and Y are the products. E) Both X and Y are the reactants.
What is needed, in addition to the parts of the Bohr model used to calculate the energies of a hydrogen atom, to understand the periodicity of the elements?
a. An understanding of the photoelectric effect. b. The calculated radii of the orbits in which electrons move. c. The principle that alkali metals (in the column of the Periodic Table containing lithium, sodium, potassium, etc.) have their outer shell of electrons filled. d. The principle that atoms have shells that each hold a certain number of electrons, with filled shells being the most stable. e. The principle that the noble gas elements accept electrons easily from other atoms.