The change in entropy when 1 kg of ice melts at 0°C is (in J/K). (Lice = 333 J/g.)
a. 335.
b. 603.
c. 1 220.
d. 1 310.
e. 2 160.
C
You might also like to view...
The element most abundant in the Sun is oxygen
Indicate whether the statement is true or false
Energy Levels: The figure shows part of the energy level diagram of a certain atom. The energy spacing between levels 1 and 2 is twice that between 2 and 3. If an electron makes a transition from level 3 to level 2, the radiation of wavelength ? is emitted. What possible radiation wavelengths might be produced by other transitions between the three energy levels?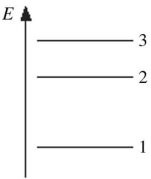
A. both ?/2 and ?/3 B. only ?/2 C. both 2? and 3? D. only 2?
In the context of studying major bodies of our solar system, what category of object does our Moon best fit?
A. jovian moon B. small asteroid C. terrestrial world D. large comet E. jovian planet
If the Q value of an endothermic reaction is –2.17 MeV, then the minimum kinetic energy needed in the reactant nuclei if the reaction is to occur must be
1.equal to 2.17 MeV. 2.greater than 2.17 MeV. 3.less than 2.17 MeV. 4.exactly half of 2.17 MeV.