Heat in the amount of 7.5 kJ is added to a closed system while its internal energy decreases by 12 kJ. How much energy is transferred as work? For a process causing the same change of state but for which the work is zero, how much heat is transferred?
What will be an ideal response?
The first law of thermodynamics tells us that
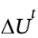 = Q + W
= Q + W
So, if Q = 7.5 kJ and
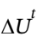 = ?12 kJ, we see that W = ?19.5 kJ. That is, 19.5 kJ was transferred from the system to the surroundings as work.
= ?12 kJ, we see that W = ?19.5 kJ. That is, 19.5 kJ was transferred from the system to the surroundings as work.
If the same change of state occurred (which tells us that 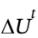 was the same), but no work was done (W = 0) we would have ?12 kJ = Q + 0. That is, if no work was done, then 12 kJ would have to be removed from the system to cause the same change in the state of the system.
was the same), but no work was done (W = 0) we would have ?12 kJ = Q + 0. That is, if no work was done, then 12 kJ would have to be removed from the system to cause the same change in the state of the system.
You might also like to view...
The roots from forests prevent _____ and help to provide clean water
A) soaking in B) runoff C) rain D) habitat
To properly set the heat anticipator, the ____ must determine what the current flow is through the heating control circuit when the furnace is operating in a steady state condition.
A. manufacturer B. homeowner C. electrician D. heating technician
What type of device is used in a typical idle air control unit?
A) DC motor B) Stepper motor C) Solenoid D) Pulsator-type actuator
How does the driver know if the electric power steering (EPS) system has a fault?
A) The vehicle will not steer at all B) A grinding noise is heard C) EPS warning light or "Power Steering" message D) Malfunction indicator light