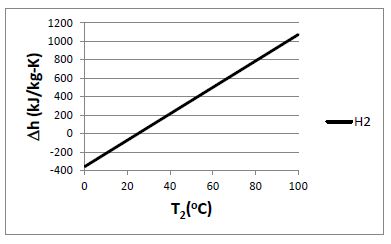
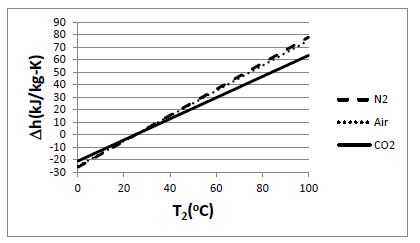
Plot the change in specific enthalpy as a function of final temperature for the following gases, each of which is initially at 25°C:
(a) hydrogen, (b) nitrogen, (c) air, (d) carbon dioxide. Assume each gas behaves as an ideal gas with constant specific heats, and consider a final temperature range of 0°C to 100°C.
Given: T1 = 25°C
What will be an ideal response?
For an ideal gas with constant specific heats: ?h = cp (T2 – T1)
For the constant-pressure specific heats: (a) H2 : cp = 14.307 kJ/kg-K; (b) N2: cp = 1.039
kJ/kg-K; (c) air: cp = 1.005 kJ/kg-K; (d) CO2: cp = 0.846 kJ/kg-K (at 300 K)
You might also like to view...
The daily report is a _____.
a. temporary written record b. record of engineering changes c. record of management tasks d. permanent written record
Postwelding cleanup, grinding, and painting add to the weldment's ____ cost.
A. process B. material C. finishing D. overhead
A problem with GIS (Geographic Information Systems) maps is that they do not recognize the curvature of the earth and cannot provide precise locations of a site on the surface of the earth
Indicate whether the statement is true or false
Fuel pressure for a certain vehicle should be about 30 p.s.i. Suppose the pressure is measured and found to be only 9 p.s.i. What percent of full pressure is present?
A) 21% B) 9% C) 27% D) None of these