In a proportion, the extremes are the two inside terms.
Answer the following statement true (T) or false (F)
False
You might also like to view...
The compounds A and B form non-ideal solutions in the liquid phase. At 50 ?C, a mixture of A and B has an EXCESS molar enthalpy as given below:
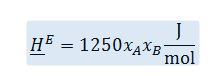
The heat capacities of the liquids are modeled as constant. Both are equal to CP=50 J/mol-K.
In a steady-state, constant pressure process, 1000 mol/min of pure A at 25 ?C is mixed with 500 mol/min of pure B at 35 ?C. The mixture then enters a heat exchanger in which the mixture is heated to 50 ?C.
A. Determine the rate at which heat is added to the heat exchanger.
B. If we are using a reference state in which the molar enthalpy, ?H, of pure A and pure B are both 0 at a temperature of 25 ?C, then what is the partial molal enthalpy, ¯H, of compound A in the mixture that is leaving the heat exchanger?
The difference between a ________________ is that one simply makes the employee aware that a problem exists while the other provides immediate negative consequences
A) Termination and separation B) Warning and reprimand C) Day off without pay and separation D) Reward and punishment
What is the estimated length of a wall that has 31 studs 16 inches on center?
A) 30 feet 4 inches B) 40 feet C) 41 feet 4 inches D) 30 feet
A heat recovery ventilation system saves energy by
A) exchanging heat between the supply and exhaust air streams B) pre-heating the ventilation supply air with electric heat strips C) pre-heating the ventilation exhaust air with electric heat strips D) decreasing the size of the indoor air conditioning system blower