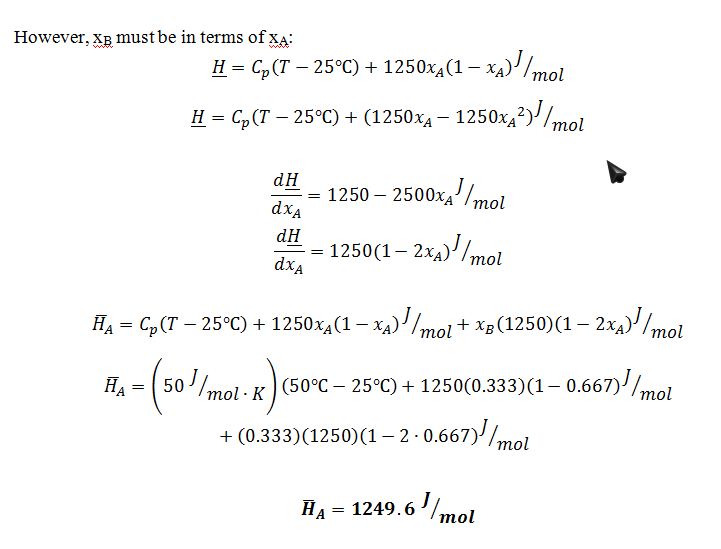
The compounds A and B form non-ideal solutions in the liquid phase. At 50 ?C, a mixture of A and B has an EXCESS molar enthalpy as given below:
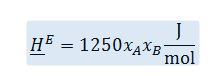
The heat capacities of the liquids are modeled as constant. Both are equal to CP=50 J/mol-K.
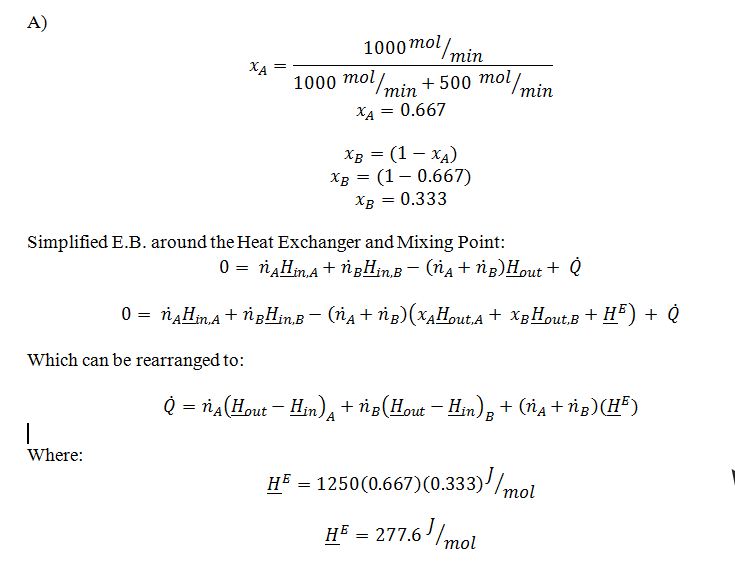
In a steady-state, constant pressure process, 1000 mol/min of pure A at 25 ?C is mixed with 500 mol/min of pure B at 35 ?C. The mixture then enters a heat exchanger in which the mixture is heated to 50 ?C.
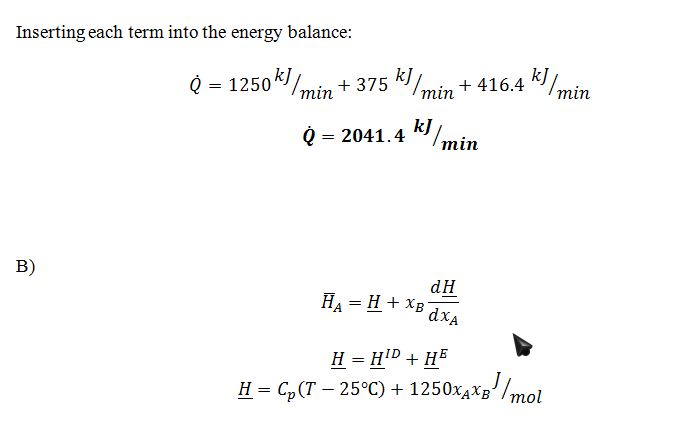
A. Determine the rate at which heat is added to the heat exchanger.
B. If we are using a reference state in which the molar enthalpy, ?H, of pure A and pure B are both 0 at a temperature of 25 ?C, then what is the partial molal enthalpy, ¯H, of compound A in the mixture that is leaving the heat exchanger?
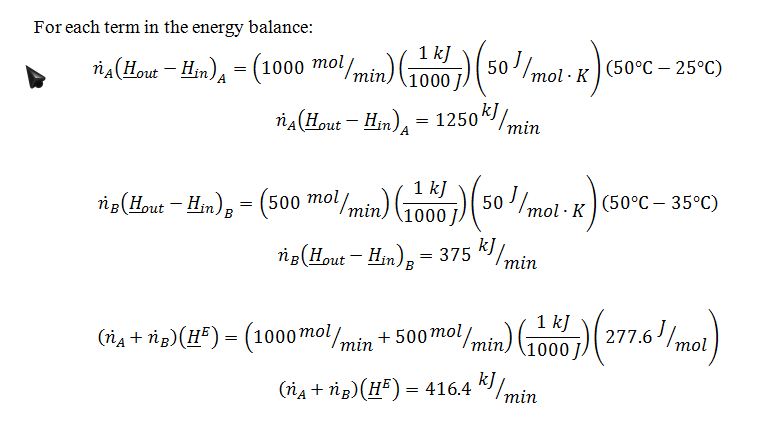
You might also like to view...
Fuel cells must produce ____________________ in order to provide electricity.
Fill in the blank(s) with the appropriate word(s).
A manufacturer of industrial grade gas handling equipment wants to have $500,000 in an equipment replacement contingency fund 10 years from now. If the company plans to deposit a uniform amount of money each year beginning now and continuing through year 10 (total of 11 deposits), what must be the size of each deposit? Assume the account grows at a rate of 10% per year.
What will be an ideal response?
The heart rates of resting neonatal dogs and cats are lower than those of adult cats and dogs
A. True B. False
Why did many small landholders in Late Republican Rome quit farming and drift with their families to the city of Rome?
a. They hoped to become clients of powerful patrons in Rome and obtain political office. b. They wanted to immigrate to Roman colonies outside of Italy. c. They could not compete against large plantation-like estates worked by imported slaves. d. They no longer wanted to work and hoped to enjoy “bread and circuses” in Rome.