Venus may have started with an ocean's worth of water. If so, where is its water now?
A) The original water remains vaporized in the atmosphere due to Venus's intense heat.
B) Most of the water is frozen beneath the surface.
C) Most of the water combined with rocks in chemical reactions.
D) The water was lost when ultraviolet light broke apart water molecules and the hydrogen escaped to space.
E) The water changed to carbon dioxide through chemical reactions.
D) The water was lost when ultraviolet light broke apart water molecules and the hydrogen escaped to space.
You might also like to view...
A Carnot refrigerator has a coefficient of performance = 2.5. The refrigerator consumes 50 W of power. How much heat is removed from the interior of the refrigerator in 1 hour?
A) 7.5 kJ B) 4.5 × 105 J C) 1.8 × 105 J D) 7.2 × 105 J E) 72 kJ
If a 20-kg object dropped in air has a terminal speed of 60 m/s, what was its acceleration at 30 m/s?
a. 9.80 m/s2 b. 7.35 m/s2 c. 4.90 m/s2 d. 2.45 m/s2 e. More information is needed to answer this question.
All the statements below are true. Which one gives the primary reason why the surface of Venus today is some 450°C hotter than the surface of Earth?
A) Venus has a much stronger greenhouse effect than Earth. B) Venus is only about 73% as far from the Sun as Earth. C) Venus has a much higher reflectivity than Earth. D) Venus has a higher atmospheric pressure than Earth.
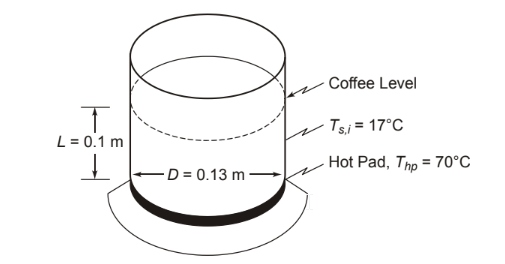
A plot of coffee has been allowed to cool to 17°C. If the electrical coffee maker is turned back on, the hot plate on which the pot rests is brought up to 70°C immediately and held at that temperature by a thermostat. Consider the pot to be a vertical cylinder 130 mm in diameter and the depth of coffee in the pot to be 100 mm. Neglect heat losses from the sides and top of the pot. How long will it take before the coffee is drinkable (50°C)? How much did it cost to heat the coffee if electricity costs $0.05 per kilowatt-hour?
GIVEN
• Coffee pot, idealized as a vertical cylinder, on a hot plate
• Initial temperature of the pot and coffee (Ts,i) = 17°C
• Hot plate temperature (Thp) = 70°C (constant)
• Pot diameter (D) = 130 mm = 0.13 m
• Depth of coffee (?) = 100 mm = 0.1 m
FIND
(a) Time for the coffee to reach 50°C (b) Cost to heat the coffee if electricity costs $0.05/kWh
ASSUMPTIONS
• Heat losses from the sides and the top are negligible
• All energy from the hot plate goes into the coffee
• Internal resistance of the coffee is negligible
• Thermal resistance of the bottom of the pot is negligible
• Coffee has the thermal properties of water
• Variation of the thermal properties of the coffee with temperature can be neglected
SKETCH
PROPERTIES AND CONSTANTS
The relevant thermal properties will be evaluated using the average coffee temperature of (17°C + 50°C)/2 = 33.5°C.
for water At 33.5°C
Density (?) = 994.6 kg/m3
Specific Heat (c) = 4175 J/(kg K) At the mean temperature of (33.5°C + 70°C)/2 = 51.8°C
Thermal expansion coefficient (?c) = 0.00047 1/K Thermal conductivity (kc) = 0.648 W/(m K) Kinematic viscosity (?c) = 0.549 × 10–6 m2/s Prandtl number (Prc) = 3.5