What element is oxidized in the following equation and what element is reduced? Sn2+ + 2 Ag ? Sn + 2 Ag?
A. The tin ion, Sn2+, is reduced, while the silver, Ag, is oxidized.
B. Both the tin ion, Sn2+, and the silver, Ag, are oxidized.
C. Both the tin ion, Sn2+, and the silver, Ag, are reduced.
D. The tin ion, Sn2+, is oxidized, while the silver, Ag, is reduced.
Answer: A
You might also like to view...
Use the hypothetical landscape and topographic map in Figure 23.2. What is the contour interval? How can you tell?
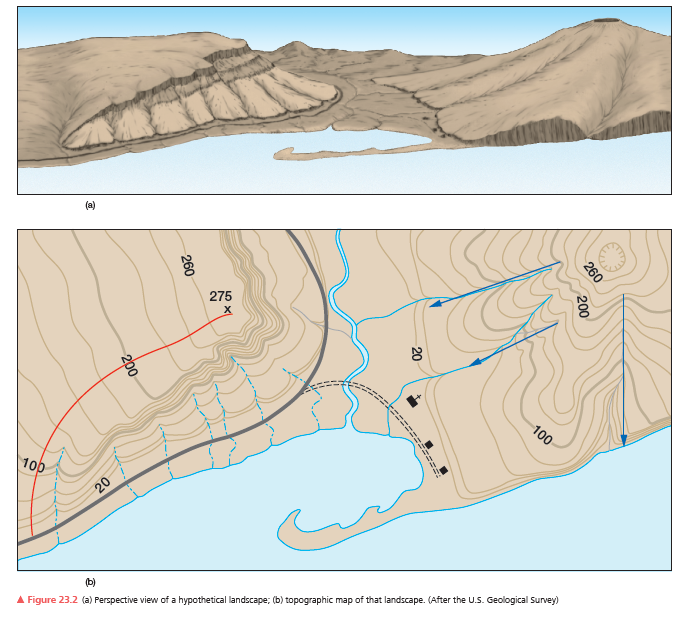
What will be an ideal response?
What environmental damage often occurs after many years of irrigation?
A) salinization B) deforestation C) air pollution D) water pollution E) acid rain
Based on insurance industry records of economic loss from natural disasters, 32 of the 40 most expensive disasters between 1970 and 2017 were ________.
A. landslides B. weather events C. volcanic eruptions D. fires E. earthquakes
The lightest form of rain is?
a. ?shower. b. ?cloudburst. c. ?virga. d. ?drizzle. e. ?fallstreak.