Two experimental runs are performed to determine the calorimetric properties of an alcohol which has a melting point of -10° C
In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20°C and the temperature at thermal equilibrium is 10°C. The specific heat capacity of water is 4190 J/kg ? K. Assume no heat is exchanged with the styrofoam container and the surroundings. What is the specific heat capacity of the alcohol? A) 1700 J/kg ? K
B) 1900 J/kg ? K
C) 2100 J/kg ? K
D) 2300 J/kg ? K
E) 2500 J/kg ? K
C
You might also like to view...
Dark dust clouds are cooler than their surroundings
Indicate whether the statement is true or false
Is peak nucleate boiling a stable boiling condition?
What will be an ideal response?
Exhibit 12-1
The figure below shows a graph of angular velocity versus time for a woman bicycling around a circular track.
?
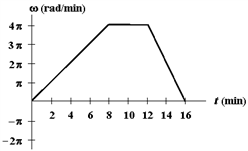
A. 4 B. 8 C. 12 D. 16 E. 32
Vision Problems: A person is nearsighted with a far point of 75.0 cm.(a) What focal length contact lens is needed to give him normal vision?(b) What is the power of the corrective lens?
What will be an ideal response?