A chemist wishes to estimate the concentration of particles in a certain suspension. She withdraws 3 mL of the suspension and counts 48 particles. Estimate the concentration in particles per mL and find the uncertainty in the estimate.
What will be an ideal response?
Let X represent the number of particles observed in 3 mL. Let 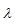 represent the true concentration in particles per mL. Then
represent the true concentration in particles per mL. Then 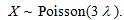 . The observed value of X is 48. The estimated concentration is
. The observed value of X is 48. The estimated concentration is 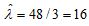 . The uncertainty is
. The uncertainty is 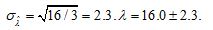
You might also like to view...
?To convert a denominator in a fraction, multiply both the numerator and the denominator by the same _____.
Fill in the blank(s) with the appropriate word(s).
What is the most popular terminal sire breed in the United States?
a. Poland China c. Chester White b. Duroc d. Landrace
Explaining to the customer that the previous technician was obviously trying to cheat them:
A) Is never a good idea. B) Will make the customer feel more trusting of you and your honesty. C) Is required by the National Service Technician's Code of Conduct. D) Is appropriate only if the technician worked for another company.
Why is environmental tobacco smoke difficult to remove from the air?
A) The majority of office workers smoke, producing large quantities of pollutants to remove. B) Tobacco smoke has an estimated 4,000 compounds in it. C) Most people cannot detect the presence of tobacco smoke in the air. D) It is hard to see.