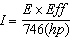What term best describes a liquid with a flash point below 140°F?
A) Corrosive B) Toxic C) Ignitable D) Radioactive
C
You might also like to view...
Does this system exhibit positive or negative deviations from Raoult’s Law? Explain your answer.
A liquid mixture of 60% by mole cyclohexanone(1) and the rest phenol (2) is in equilibrium with its vapor at 144°C. At this temperature, the vapor pressure of the cyclohexanone is 0.752 bar and that of the phenol is 0.3166 bar. Note that the system does form an azeotrope at this temperature whose composition is 29.4% by mole cyclohexanone and whose pressure is 0.261 bar. Given the above information, estimate the equilibrium pressure and vapor-phase composition of this system.

____ energy involves tapping underground reservoirs in volcanically active areas
A) Geothermal B) Hydroelectric C) Ground D) Natural
Determine i(t) for t > 0 in the circuit of Fig. 8.96.
 FIGURE 1.png)
Solve the equation for the current (I) required by a motor when the horsepower, percent efficiency of the motor, and the voltage are known.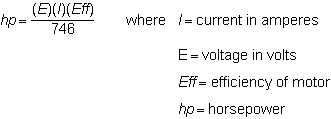
A. ![]()
B. 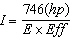
C. 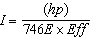
D.