A 10-L flask and a 1-L flask each contain two moles of ideal diatomic gas (but not the same gas) at 25°C. Which of the following statements about these gases must be true? (There could be more than one correct choice.)
A) The internal (thermal) energy of the gas in both flasks is the same.
B) The internal (thermal) energy of the gas in the larger flask is greater than the internal (thermal) energy of the gas in the smaller flask.
C) The internal (thermal) energy of the gas in the smaller flask is greater than the internal (thermal) energy of the gas in the larger flask.
D) The molecules in the larger flask have the same root-mean-square speed as those in the smaller flask.
E) The molecules in the smaller flask have the same average kinetic energy per molecule as those in the larger flask.
A, E
You might also like to view...
Series/Parallel Circuits: Draw a circuit consisting of a battery connected to two resistors, R1 and R2, in series with each other and a capacitor C connected across the resistors.
A. 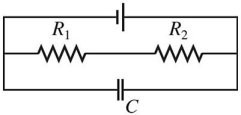
B. 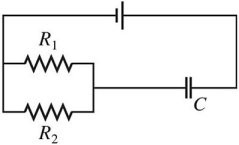
C. 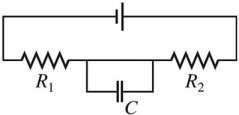
D. 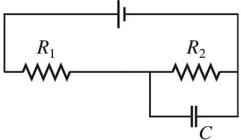
The vibrational direction of an electron and the plane of polarization of the light it emits
A) are the same. B) are at right angles to each other. C) may or may not be at right angles to each other. D) are independent of each other.
If the radius of the electron orbit in the n = 1 level of the hydrogen atoms is 0.052 9 nm, what is its radius for the n = 4 level? (Assume the Bohr model is valid)
a. 0.212 nm b. 0.045 nm c. 0.716 nm d. 0.846 nm e. 0.920 nm
An object will ______________ in a fluid if its average density is ______________ the density of the fluid
a. sink; greater than b. float; equal to c. sink; less than d. float; twice