If the radius of the electron orbit in the n = 1 level of the hydrogen atoms is 0.052 9 nm, what is its radius for the n = 4 level? (Assume the Bohr model is valid)
a. 0.212 nm
b. 0.045 nm
c. 0.716 nm
d. 0.846 nm
e. 0.920 nm
d
You might also like to view...
Radioactivity Basics: A nuclear reaction is shown: 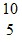 B +
B + 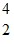 He ?
He ? 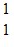 H + ? . Which one of the following isotopes is the missing nuclear product?
H + ? . Which one of the following isotopes is the missing nuclear product?
A. 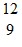 F
F
B. 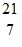 N
N
C. 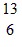 C
C
D. 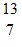 N
N
E. 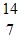 N
N
When a baseball bat hits the ball, the impulse delivered to the ball is increased by
A. The impulse cannot be changed. B. rapidly stopping the bat after impact. C. letting the bat recoil upon impact. D. "follow through" on the swing, which increases contact time.
If m = 4, how may possible values can the spin quantum number ms have?
A) 3 B) 5 C) 4 D) 2 E) 1 F) 0
An observer moving with a light clock in a spaceship sees a light flash bouncing up and down between parallel mirrors in 1 nanosecond. An observer at rest outside the spaceship sees the same up-and-down flash in
A) 1 nanosecond also. B) less than 1 nanosecond. C) more than 1 nanosecond.