Discuss the languages of North Africa and Southwest Asia, paying special attention to the non-Arab languages in the region
What will be an ideal response?
While Semitic languages, especially Arabic (and to a lesser extent, Hebrew) are dominant, other languages are present in the region. In fact, in the two countries in the region that have never been occupied (Iran and Turkey), non-Semitic languages are the rule. Turks speak an Altaic language while Iranians speak an Indo=European language.
You might also like to view...
A 500-N skydiver reaches terminal velocity at 90 km/h, with an air resistance of
A. 250 N. B. 410 N. C. 500 N. D. 90 N.
Using the graph below, construct a topographic profile from Point A to Point B. Plot index contours, and any intermediate contours necessary to accurately show significant changes in topography. The vertical exaggeration of the profile is 2x. The stereogram may be helpful in recognizing the extent of some terrace levels.
The following questions are based on Figure 49-8, a portion of the “San Clemente Island Central, California” quadrangle shown on the following page (scale 1:24,000; contour interval 25 feet; index contours drawn every fourth line; to view this map in color, go to the Lab Manual website or scan the QR code for this exercise), and the stereogram of San Clemente Island (Figure 49-7). The map and stereogram show a series of marine terraces on San Clemente Island in southern California (32°51"08"N, 118°29'58"W). The terraces have been incised by streams in several places.
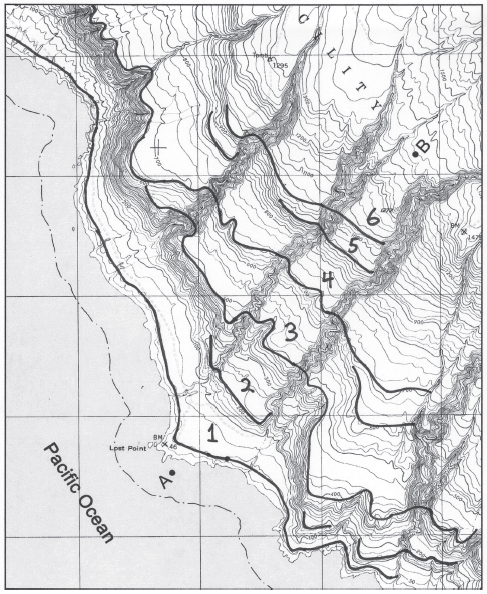
Figure 49-8: USGS “San Clemente Island Central, California” quadrangle (scale 1:24,000; contour interval 25 feet; note that the index contours on this map have been drawn every fourth line;cN)
Why is the formation of iron hydroxide, Fe(OH)2, from Fe2+ and OH- not considered an oxidation-reduction reaction?
A) The iron is only oxidized and nothing is reduced. B) The iron is only reduced, not oxidized. C) The Fe2+ ion is not changed during this chemical reaction. D) Iron can never be oxidized or reduced.
By definition, inorganic fertilizers do not contain ________
A) either nitrogen or phosphorus B) phosphorus C) nitrogen D) carbon