How many nanometers are there in one meter?
Figure
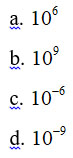
Answer: b
You might also like to view...
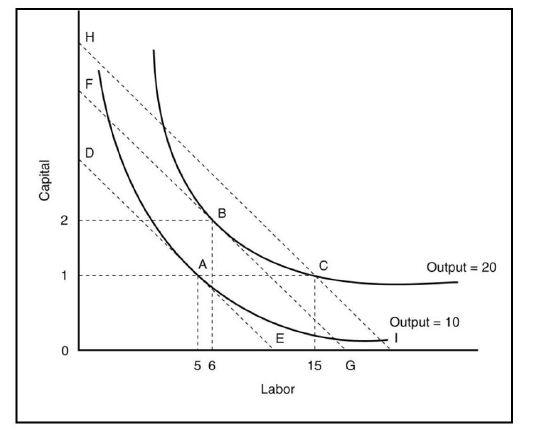
In the graph below, given iso-cost line DE, the producer can maximize profits by producing ________ units of output, using ________ units of capital and ________ units of labor.
This problem concerns vapor-liquid equilibrium mixtures of compounds Q and W. We know that:
• The vapor pressure of pure compound Q is 0.5 bar at 300 K. • The vapor pressure of pure compound W is 1.0 bar at 300 K. A) If a liquid is composed of 40% Q and 60% W and at 300 K, give your best estimate of the bubble pressure and composition of the first bubble of vapor that WOULD be observed IF Q and W formed ideal liquid solutions. Q and W do not, in reality, form ideal solutions. In reality, for vapor liquid equilibrium at 300 K, there is an azeotrope at P=0.9 bar and T=300 K, in which the liquid and vapor phase both contain 40% Q. Use this fact—as well as the vapor pressure data given above—to answer questions B and C. B) Give your best estimate of the activity coefficients of Q and W in the azeotropic mixture at 300 K. C) Give your best estimate of the bubble pressure and composition of the first bubble of vapor in equilibrium with a liquid that is composed of 75% Q and 25% W at 300 K.
Which of the following transfers heat most efficiently to the engine coolant?
A. parent bore B. dry liners C. wet liners D. oversized dry liners
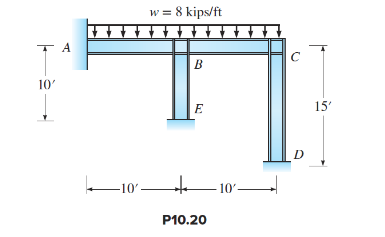
Analyze the frame in Figure P10.20. Compute all reactions. Given: EI is constant.