The room-temperature yield strength of very-low-carbon steel as a function of the square root of the interstitial solid-solution carbon concentration is shown in Figure 7.12.
(a) Evaluate all of the constants in the equation for the tensile yield strength as a function of solid-solution chemical composition.
(b) The maximum equilibrium composition of interstitial carbon possible in ferritic iron is 0.0011 atom fraction (af). If an iron-carbon alloy of this composition could be produced at room temperature, what should the yield strength be? You must solve this problem analytically; you can check your result graphically.
(c) If the composition of 0.0011 af of carbon is exceeded, the extra carbon forms iron carbide. If a specimen is produced with 0.0015 af of carbon, do you expect the strength to be given by an extension of the line in the figure? Briefly explain your answer.
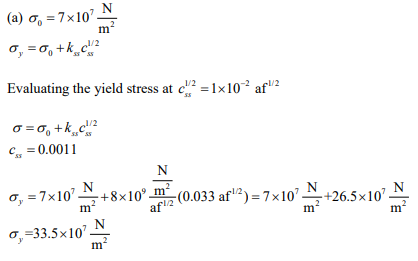
(c) No, the strength with precipitates should not have the same dependence upon composition as the solid solution.
You might also like to view...
Inertia is defined as a
A) force. B) property of matter. C) change in motion. D) none of the above
Matter Waves: A proton has a speed of 7.2 × 104 m/s. What is the energy of a photon that has the same wavelength as the de Broglie wavelength of this proton? (melectron = 9.11 × 10-31 kg, c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ? s)
A. 230 keV B. 150 keV C. 300 keV D. 370 keV E. 440 keV
What is meant by proper time?
What will be an ideal response?
The type of current supplied by a battery is __________
Fill in the blank(s) with correct word