A liquid has a constant CP=100 J/mol•K. 1 mole of this liquid is confined in a piston-cylinder device, with an initial temperature of T=500 K and pressure P=1 bar. The insulation is removed from the cylinder, allowing it to exchange heat with the surroundings, which can be modeled as a heat reservoir at T=300 K and P=1 bar. The process continues until the liquid in the cylinder reaches equilibrium with the surroundings.
A) Find the change in entropy of the liquid resulting from this process.
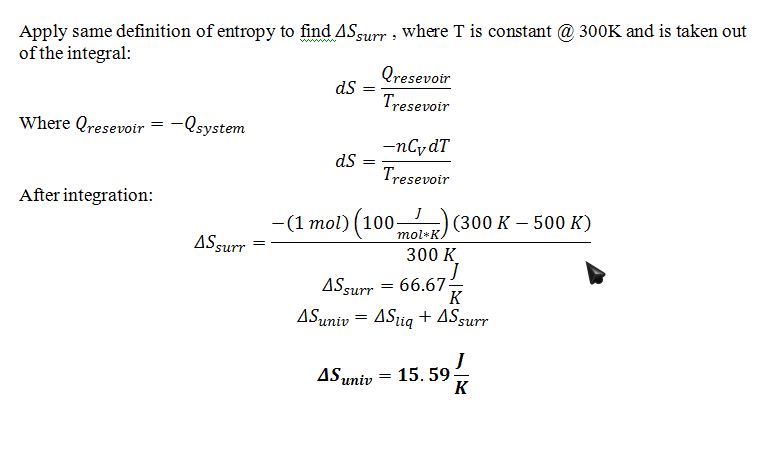
B) Find the change in entropy of the universe resulting from this process.
When equilibrium is reached, the final temperature of the liquid will be 300 K for thermal equilibrium with the heat reservoir.
Apply the definition of change in entropy for the liquid. The heat transfer is not reversible, but we use a reversible path to calculate the state property dS:
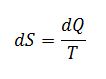
Where dQ is found through the energy balance of the process:
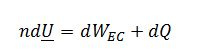
Where dWEC is negligible in this case assuming the liquid is incompressible (dW_EC= -PdV)

Note: The reversible and irreversible paths will have the same Q, because it is equal to the change in U which is a state property. If there was a significant expansion/contraction work term in the energy balance, the reversible and irreversible Q values wouldn’t be the same.
After substitution:
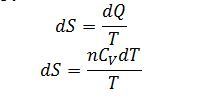
Substitute dQ into definition of entropy:
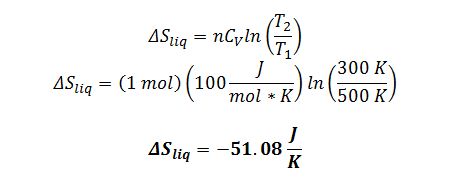
After integration where n and CV are treated as constants:
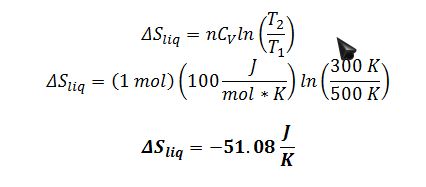
You might also like to view...
An average home in the United States consumed about 12,773 kWh of electricity in 2005. The national average carbon dioxide emission rate for electricity generated in 2007 was 1,293 lbs. of CO2 per megawatt-hour. Approximately 7% of the electricity generated is wasted due to losses in the transmission and distribution system.
a. How much CO2 was generated per year for an average U.S. home due to electricity use? b. How much CO2 was emitted from household use of electricity is there were 111.1 million homes in the United States in 2005? c. How much CO2 will be emitted from household use of electricity if there are 166 million homes in the United States in 2050? What will be an ideal response?
T
Indicae wheher he saemen is rue or false
Which one of the following describes the left to right order of a feature control frame?
A. tolerance zone descriptor, geometric characteristic, geometric characteristic tolerance, primary datum reference B. geometric characteristic tolerance, geometric characteristic, tolerance zone descriptor, datum reference C. geometric characteristic symbol, tolerance zone descriptor, geometric characteristic tolerance, datum reference D. geometric characteristic symbol, material condition symbol, geometric characteristic tolerance, tolerance zone descriptor, multiple datum reference E. none of the above
A midcareer engineer hopes to have $2 million available for his use when he retires 20 years from now. He plans to deposit a uniform amount semiannually, beginning now and every 6 months thereafter through the end of year 20. If his investment account has a yearly return of 8% per year, compounded quarterly, the interest rate, i, that must be used in the A?F equation to determine the size of the uniform deposits is:
(a) 2% (b) 8.24% (c) 8.00% (d) 4.04%