Two 0.95-kg masses, one aluminum and one tin, both at 80.0°C, are placed in 1.00 kg of water at 20.0°C. What is the equilibrium temperature of the system? The specific heats of aluminum and tin are 900 J/kg/°C and 227 J/kg/°C, respectively
a. 32.2 °C
b. 27.1 °C
c. 13.2 °C
d. 40.3 °C
a
You might also like to view...
Simple Harmonic Motion: A sewing machine needle moves in simple harmonic motion with a frequency of 2.5 Hz and an amplitude of 1.27 cm.(a) How long does it take the tip of the needle to move from the highest point to the lowest point in its travel?(b) How long does it take the needle tip to travel a total distance of 11.43 cm?
What will be an ideal response?
Compute the average emittance of anodized aluminum at 100°C and 650°C from the spectral curve. Assume ?? = 0.8 for ? > 9 ?m
GIVEN
The spectral curve for anodized aluminum
FIND
The average emittance (?) at (a) 100°C = 373 K, and, (b) 650°C = 923 K
SKETCH
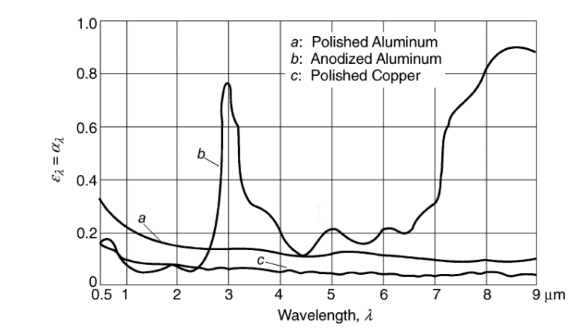
PROPERTIES AND CONSTANTS
the Stephan-Boltzmann constant (?) = 5.67 10–8 W/(m2 K4)
Polarization: Unpolarized light passes through three ideal polarizing filters. The first filter is oriented with a horizontal transmission axis, the second one has its transmission axis at 30° from the horizontal, and the third filter has a vertical transmission axis. What percent of the light gets through this combination?
A. 9.4% B. 91% C. 50% D. 0% E. 33%
What does the Grashof number mean?
What will be an ideal response?