Molecular Speeds: Oxygen molecules are 16 times more massive than hydrogen molecules. At a given temperature, how do their average molecular speeds compare? The oxygen molecules are moving
A. four times faster than the hydrogen molecules.
B. at 1/4 the speed of the hydrogen molecules.
C. sixteen times faster than the hydrogen molecules.
D. at 1/16 the speed of the hydrogen molecules.
E. at 1/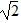 the speed of the hydrogen molecules.
the speed of the hydrogen molecules.
Answer: B
You might also like to view...
Superposition of waves can occur
a. in transverse waves. b. in longitudinal waves. c. in sinusoidal waves. d. in all of the above. e. only in (a) and (c) above.
Heteroatoms make a difference in the physical and chemical properties of an organic molecule because
A) they add extra mass to the hydrocarbon structure. B) each heteroatom has its own characteristic chemistry. C) they can enhance the polarity of the organic molecule. D) all of the above
Photoelectric Effect: When it is struck by 240-nm photons, a metal ejects electrons with a maximum kinetic energy of 2.58 eV. What is the work function of this material? (c = 3.00 × 108 m/s, h = 6.626 × 10-34 J ? s, 1 eV = 16.0 × 10-19 J)
A. 2.60 eV B. 2.18 eV C. 3.02 eV D. 3.43 eV
An ice cube is floating in a glass of water with the water level just reaching the top of the glass. If the temperature in the surrounding area is above freezing, the ice cube will melt. When it does,
A. the water level in the glass will remain unchanged as the ice continues to melt. B. the water level in the glass will begin to go down. C. water will begin to spill over the top of the glass. D. it is impossible to tell what the water level will do from the information given.