Uncertainty Principle Using Momentum and Position: You measure the velocity of an electron to an accuracy of ±22.6 m/s. What is the minimum uncertainty in its position? (h = 6.626 × 10-34 J ? s, melectron = 9.11 × 10-31 kg)
A. 8.05 ?m
B. 4.03 ?m
C. 16.1 ?m
D. 32.2 ?m
E. 5.12 µm
Answer: E
You might also like to view...
A homeowner has a new oil furnace which has an efficiency of 60%. For every 100 barrels of oil needed to heat his house, how much (in barrels of oil) goes up the chimney as waste heat?
A. 20 B. 60 C. 40 D. 80 E. 10
Suppose you drop a 10-pound weight and a 5-pound weight on the Moon, both from the same height at the same time. What will happen?
A) Both weights will float freely, since everything is weightless on the Moon. B) Both will hit the ground at the same time. C) The 10-pound weight will hit the ground before the 5-pound weight. D) The 5-pound weight will hit the ground before the 10-pound weight.
The figure below shows a planet traveling in a clockwise direction on an elliptical path around a star located at one focus of the ellipse. When the planet is at point A,
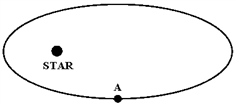
a.
its speed is constant.
b.
its speed is increasing.
c.
its speed is decreasing.
d.
its speed is a maximum.
e.
its speed is a minimum.
A diffraction grating with 10 rulings is illuminated by a light source. When do other wavelengths begin to distinguish themselves from 500 nm in the second order?
1.at 475 nm and 525 nm 2.at 450 nm and 550 nm 3.at 495 nm and 505 nm 4.at 487.5 nm and 512.5 nm