Consider two cylinders of gas identical in all respects except that one contains oxygen O2 and the other helium He
Both cylinders initially contain the same volume of gas at 0°C and 1 atm of pressure and are closed by a movable piston at one end. Both gases are now compressed adiabatically to one-third their original volume.
(a) Which gas will show the greater temperature increase?
A. the O2
B) the He
C) Neither; both will show the same increase.
D) It is impossible to tell from the information given.
(b) Which gas will show the greater pressure increase?
A. the O2
B) the He
C) Neither; both will show the same increase.
D) It is impossible to tell from the information given.
(a) B (b) B
You might also like to view...
Suppose that the energy difference between adjacent energy levels for an oscillator in an Einstein solid is ? = 0.005 eV. We would expect the average energy of this oscillator when it is in contact with a reservoir at room temperature to be
A. negligible.
B. smaller than 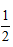 kBT.
kBT.
C. about equal to 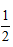 kBT.
kBT.
D. about equal to kBT.
E. significantly larger than kBT.
F. One doesn’t have enough information to tell.
Two long straight parallel wires separated by a distance of 20 cm carry currents of 30 A and 40 A in opposite directions. What is the magnitude of the resulting magnetic field at a point that is 15 cm from the wire carrying the 30-A current and 25 cm from the other wire?
A. 51 ?T B. 33 ?T C. 72 ?T D. 64 ?T E. 46 ?T
An inertial force can be felt by observers
A. in a vacuum. B. at rest in any medium. C. moving much more slowly than the speed of light. D. whose reference frame moves in a circle.
The average kinetic energy of a nitrogen molecule at room temperature (20°C) is
a. 2 × 10^?21 J. b. 4 × 10^?21 J. c. 6 × 10^?21 J. d. 8 × 10^?21 J. e. 1 × 10^?20 J.