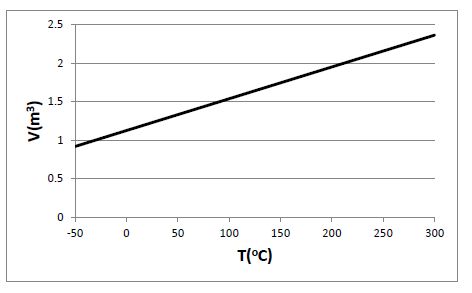
Plot the total volume as a function of temperature for 0.5 kg of hydrogen gas at 500 kPa, for temperatures varying between -50°C and 300°C. Assume the hydrogen behaves as an ideal gas.
Given: m = 0.5 kg; P = 500 kPa; H2 gas
What will be an ideal response?
From the ideal gas law, V = mRT/P, with R = 4.124 kJ/kg-K
Remember to convert the temperature to Kelvin for the calculations.
You might also like to view...
How can the estimator get an estimate of the depth of topsoil on the project?
What will be an ideal response?
The length of the growing season varies according to the:
a. amount of the 10-year average rainfall b. number of days of winds higher than 15 mph c. depth of the seed planted d. area of the country in which you live
Technician A says that a PTO driveshaft should be made of a solid steel bar when the auxiliary it drives has to be driven at a speed greater than 1200 rpm. Technician B says that solid PTO driveshafts can be made from round section, square section, and hex section steel stock. Who is correct?
A. Technician A only B. Technician B only C. Both A and B D. Neither A nor B
A low-velocity fastening system that is used to drive steel pins or threaded studs into masonry and steel is a(n)
_____. a. pneumatic screw nailer b. powder-actuated tool c. air impact wrench d. mini hammer drill