Compute the final pressure when the substance is heated from 15°C and 1 bar
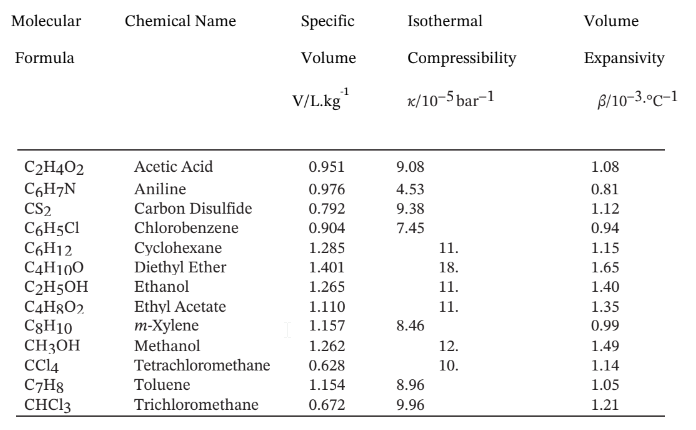
The specific volume, isothermal compressibility, and volume expansivity of several liquids at 20°C and 1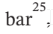 where ? and ? may be assumed constant.
where ? and ? may be assumed constant.
Volumetric Properties of Liquids at 20°C
First ethanol will be chosen as the substance to be used. In the problem the volume is held constant, both the temperature and pressure come into play here. We start with the equation
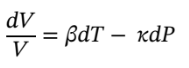
At constant volume, it becomes
0 = ?dT ? ?dP
Integrating

and solving for  gives
gives
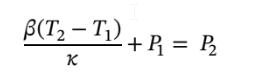
Plugging in the values gives
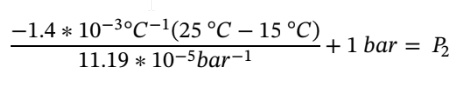
Leading 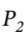 = 126.11 bar
= 126.11 bar
You might also like to view...
What is the equilibrium composition of this system at that T and P if you start with a 1 mole of H2O and 2 moles of CO? Assume the heat of reaction is not a function of temperature.
The “water-gas” shift reaction is catalyzed by an iron oxide/chromium oxide catalyst at 700 K and 2 bar. H2O + CO ? CO2 +H2
Combination fittings can be used _____
Fill in the blank(s) with the appropriate word (s).
Explain why it is important to investigate the uses and limitations of each type of thermometer before selecting the instrument to be used.
What will be an ideal response?
____ are called depletion mode devices.
A. P-channel JFETs B. P-type units with P channels C. N-channel JFETs D. N-type units with N channels