A cylinder containing an ideal gas has a volume of 2.6 m3 and a pressure of 1.5 × 10^5 Pa at a temperature of 300 K. The cylinder is placed against a metal block that is maintained at 900 K and the gas expands as the pressure remains constant until the temperature of the gas reaches 900 K. The change in internal energy of the gas is +6.0 × 10^5 J. How much heat did the gas absorb?
a. 0
b. 3.4E+5 J
c. 6.0E+5 J
d. 1.4E+6 J
e. 7.8E+5 J
d
You might also like to view...
Calculate the concentration of vacancies in silicon at the solid-liquid equilibrium temperature of 1683 K where single crystals of silicon are grown. The enthalpy of formation for vacancies in silicon is 2.3 eV/vacancy.
What will be an ideal response?
The galactic center lies in the direction of which constellation?
A) Orion B) the Big Dipper C) Leo D) Sagittarius E) Taurus
A block is pushed across a rough horizontal surface from point A to point B by a force (magnitude P = 5.4 N) as shown in the figure. The magnitude of the force of friction acting on the block between A and B is 1.2 N and points A and B are 0.5 m apart. If the kinetic energies of the block at A and B are 4.0 J and 5.6 J, respectively, how much work is done on the block by the force P between A and B?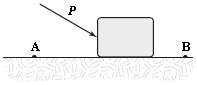
A. 2.7 J B. 1.0 J C. 2.2 J D. 1.6 J E. 3.2 J
The ozone layer blocks much of the Sun's ________ radiation
A) gamma ray B) X-ray C) radio D) infrared E) ultraviolet