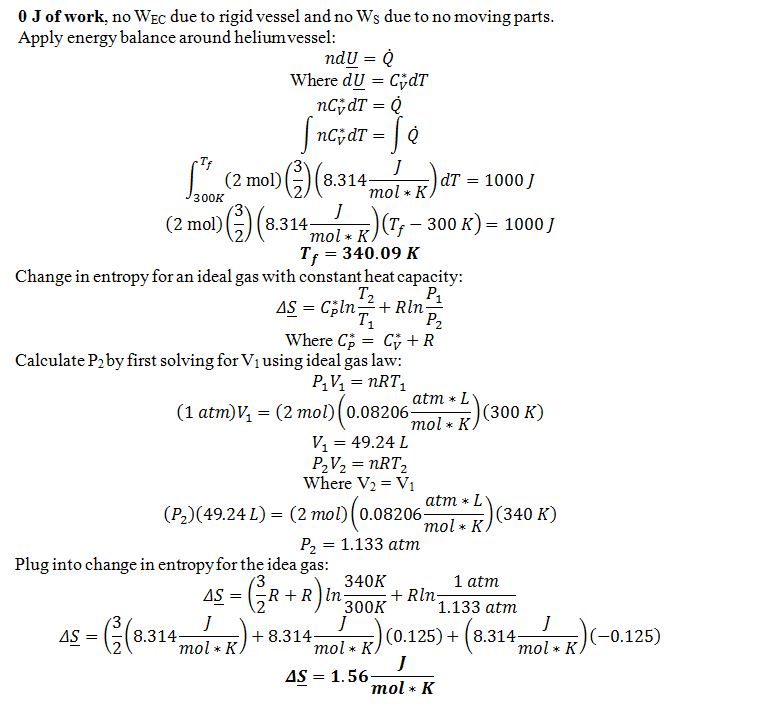
Two moles of helium, which you can model as an ideal gas with Cv*= (3/2)R, is confined in a rigid vessel (constant volume) that is sealed and has no interior moving parts. Initially, the gas has P=1 atm and T=300 K. If 1000 Joules of heat are added to the vessel, determine:
A) The amount of work added to, or removed from, the helium.
B) The final temperature of the helium.
C) The change in entropy of the helium. (If you have no answer to part B, assume the final temperature is T=400 K).
You might also like to view...
What are some of the downsides to manual operation?
What will be an ideal response?
Explain the purpose of the General and Supplementary Conditions to the Contract.
What will be an ideal response?
These springs are arched or bent shapes designed so that when placed in machinery, they cause tension on adjacent parts.
a. compression spring b. extension spring c. torsion spring d. flat spring
The non recourse loan:
a. Has a loan rate that is the interest rate on the loan. b. Is a means of price support. c. Provides that the government will take over the commodity in full payment of the loan in the event the farmer does not sell the commodity. d. All of the above. e. b and c above.