A piece of metal at 80°C is placed in 1.2 L of water at 72°C. The system is thermally isolated and reaches a final temperature of 75°C. Estimate the approximate change in entropy for this process
What will be an ideal response?
0.2 cal/°K
You might also like to view...
A 160 lb person has a mass of 160/32 = 5 slugs. A 160-lb person steps into an elevator and stands on a bathroom scale. Determine the scale reading for the given scenarios. The elevator cable breaks
a. 150 lb b. 160 lb c. 170 lb d. none of the above
Starting from rest, a 2 kg body acquires a speed of 8 m/s in 2 seconds. The net force acting on the body is
a. 2 N b. 4 N c. 8 N d. 16 N e. 6 N
How would the inward migration of a Jovian-like planet in an extrasolar planetary system alter the probability of Life appearing?
A) it would improve chances since it would protect inner, Terrestrial-like planets from impacts B) it would improve chances slightly since some of the Terrestrial planets may become moons of the Jovian planet C) it would greatly decrease the chance since the orbits of the inner, Terrestrial-like planets would be disrupted D) it would make no difference at all
Determine the equivalent capacitance of the combination shown when C = 12 pF
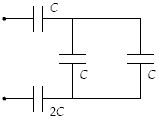
a.
48 pF
b.
12 pF
c.
24 pF
d.
6.0 pF
e.
59 pF