3.0 kg of air is initially at 200 kPa and 10oC. The air is compressed in a polytropic process, following the relationship PVn = constant. The air is compressed until the volume is 0.40 m3. Determine the work done and the final temperature of the air for values of n of 1.0, 1.1, 1.2, 1.3, and 1.4, and plot the work as a function of n.
Given: m = 3.0 kg; P1 = 200 kPa; T1 = 10oC = 283 K; V2 = 0.40 m3
What will be an ideal response?
For air, R = 0.287 kJ/kg-K
From the ideal gas law, V1 = mRT1/P1 = 1.218 m3
For a polytropic process with n = 1: 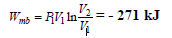
For a polytropic process with n ? 1: 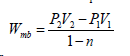
To solve for P2, use P2 = P1 (V1/V2)n
The following values can be found from these two equations:
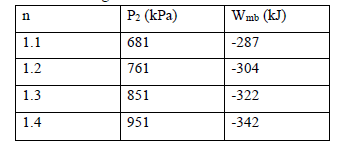
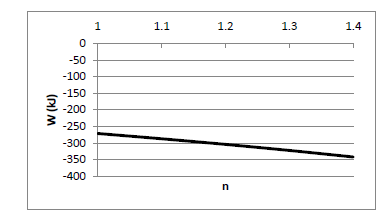
You might also like to view...
?Natural rubber is very sensitive to temperature, but Charles Goodyear found that by kneading ____ into it improved its characteristics.
A. ?powdered horn B. ?gutta percha C. ?powdered sulfur D. ?shellac
Valves can be checked for proper operation by using what test(s)?
A) Vacuum test B) Free fall test C) Wet air test D) All of the above
A commercial project requires the installation of 127 square yards of carpet. Using a productivity rate of 0.108 labor hours per square yard and a productivity factor of 1.05, determine the number of labor hours required to install the carpet.
What will be an ideal response?
?The wall thickness for tubing is measured in ____________.
A. ?thickness in inches B. ?inches or U.S. standard sheet metal gauge C. ?pressure range D. ?thickness in millimeters