A sealed 89-m3 tank is filled with 6000 moles of oxygen gas (O2 ) at an initial temperature of 270 K. The gas is heated to a final temperature of 350 K
The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol • K = 0.0821 L • atm/mol • K. The initial pressure of the gas is closest to
A) 0.15 MPa.
B) 0.17 MPa.
C) 0.19 MPa.
D) 0.13 MPa.
E) 0.11 MPa.
A
You might also like to view...
Why is the image seen in astronomical telescope eyepieces inverted?
What will be an ideal response?
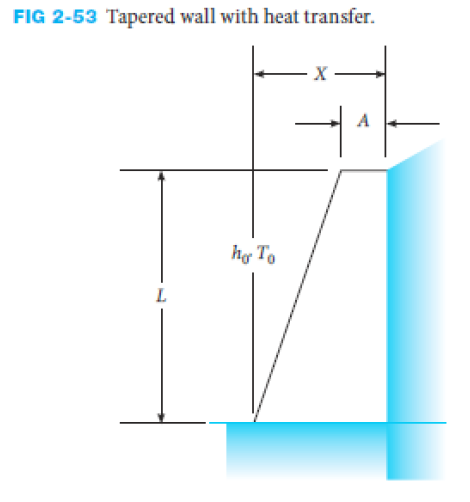
Write the governing equation and the necessary boundary conditions for the problem of a tapered wall as shown in Figure 2-53.
A solid cylinder with a radius of 10 cm and a mass of 3.0 kg is rotating about its center with an angular speed of 3.5 rad/s. What is its kinetic energy?
A) 0.18 J B) 0.092 J C) 0.96 J D) 1.05 J E) 0.53 J
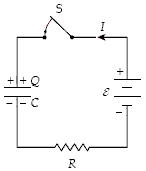
At t = 0 the switch S is closed with the capacitor uncharged. If C = 50 ?F, ? = 20 V, and R = 4.0 k?, what is the charge on the capacitor when I = 2.0 mA?
a.
360 ?C
b.
480 ?C
c.
240 ?C
d.
600 ?C
e.
400 ?C