10 moles of an ideal gas is confined in a piston-cylinder device. The gas is initially at T = 300 K and P = 1 bar. It is compressed isothermally to 10 bar. The gas has a heat capacity CV = (5/2)R.
A) What is the final volume of the gas?
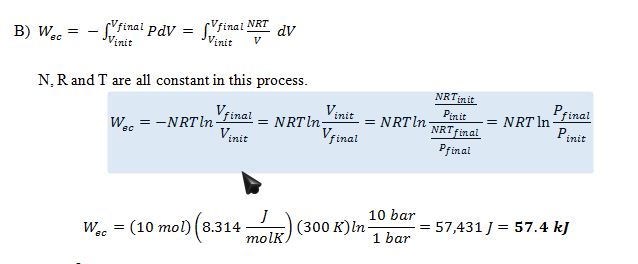
B) How much work is required to accomplish the compression?
C) What is the change in internal energy of the ideal gas?
D) What is the change in enthalpy of the ideal gas?


You might also like to view...
Using both the ideal gas law and van der Waals equation, determine the pressure of N2 that has a specific volume of 0.95 m3/kg and a temperature of 350 K.
Given: N2, v = 0.95 m3/kg; T = 350 K. What will be an ideal response?
Why is it important to provide shelter for pigs?
What will be an ideal response?
The Seebeck effect occurs when pressure is applied to a crystal so that the crystal deforms and produces a small voltage
Indicate whether the statement is true or false
Which of the following best describes the normal logical operation of a NOR gate latch?
A) Inactive SET and CLEAR inputs = 1, active SET input = 0, active CLEAR input = 1 B) Inactive SET and CLEAR inputs = 1, active SET input = 1, active CLEAR input = 0 C) Inactive SET and CLEAR inputs = 0, active SET or CLEAR inputs = 1 D) Inactive SET and CLEAR inputs = 0, active SET or CLEAR inputs = 0